Human embryonic stem (hES) cells were developed in 1998 for potential use in regenerative medicine. However, issues such as rejection of hES cells after transplantation and the use of human embryos have become impediments to their development and clinical use. Dr. Shinya Yamanaka, Kyoto University, discussed his work on the induction of pluripotency in somatic cells to yield induced pluripotent stem (iPS) cells.
In 2000, Dr. Yamanaka’s laboratory initiated a study to reprogram somatic cells to become ES-like stem cells using pluripotency maintenance factors. The first step was to identify pluripotency maintenance factors suitable for this purpose. At the time it was known that LIF, STAT3, Sox2, and Oct3/4 were critical transcription factors in ES cells. Dr. Yamanaka’s laboratory began searching for other important transcription factors in ES cells and in 2003-2004 identified several candidate factors. Together with factors found by other groups, a total of 24 transcription factors were identified.
A sensitive assay system was needed to evaluate candidate factors. Fbx15 is specifically expressed in ES cells and is activated by Oct3/4 and Sox2. Fbx15 knockout (KO) mice were created by replacing Fbx15 with the neomycin gene (NeoR). The KO mice were healthy, so another approach was used. Somatic cells from these mice were G418 sensitive due to the lack of Fbx15. ES cells expressed Fbx15 and were strongly resistant to G418. Mouse embryonic fibroblasts from the KO mice were used to make a sensitive assay.
The new assay was used to evaluate the 24 candidate genes, which were introduced into neonate fibroblasts via retroviral vectors. The factors - Oct3/4, Sox2, Klf4, and c-Myc - gave rise to drug resistant colonies, implying potential pluripotency.
|
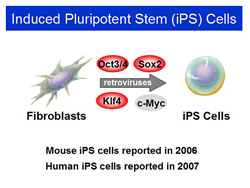
Figure 1. Induced Pluripotent Stem (iPS) Cells.
【Click to enlarge】 |
|
|
Figure 2. Applications of iPS Cells.
【Click to enlarge】 |
|
|
In 2006, Dr. Yamanaka’s lab announced that ES-like stem cells were generated by introducing the four transcription factors into mouse fibroblasts. Dr. Yamanaka named the ES-like cells induced pluripotent stem cells, or iPS cells (Figure 1). In 2007, the group also reported the generation of human iPS cells.
The iPS cells had similar morphology and proliferation markers to ES cells. Using differentiation techniques from work with ES cells, a variety of cell types can be produced from the iPS cells, including cardiac myocytes.
iPS cells have enormous potential for clinical application (Figure 2).
Regenerative medicine such as cell therapies is one possibility in the future if the clinical safety has been firmly established and other problems have been solved. For example, cardiac cells can be generated from iPS cells and may be used for cell tansplant therapy in patients with cardiac disease, and iPS cell-derived neural cells may be used to treat neurological conditions such as Parkinson’s disease. These cells are generated from the patient’s own fibroblasts and carry the patient’s own genes. They may be transplanted back into the patient without the potential for immune rejection.
The iPS cells can also be used for in vitro toxicity testing. In one experiment, a cholinergic agonist was administered to cardiomyocytes derived from wild-type and Akap10 mutant ES cells. Mild bradycardia occurred in the wild-type cells, while significantly amplified bradycardia was observed in the Akap10 mutant cells. In humans, iPS cells can be generated from an individual and tested in vitro for cardiac toxicity. For example, some drugs cause long QT syndrome in some patients, resulting in lethal arrhythmia. In vitro testing with iPS cells may be able to predict which patients will experience long QT syndrome when administered a particular drug. Another application is the establishment of disease models for drug screening and development. Due partly to the lack of disease models, effective treatments do not exist for motor neuron diseases such as spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS). Researchers are currently working on disease models of SMA and ALS using iPS cells from patients to generate motor neurons carrying the mutant disease-causing genes. Work with the patient-derived iPS cells led to the finding that SMA is associated with abnormal motor neurons, while ALS neurons appear to be normal. SMA is a monogenic disease that is primarily genetic in origin, while ALS is primarily influenced by environmental factors and aging. An important task for future research will be to replicate an in vitro model of ALS. At Kyoto University’s Center for iPS Cell Research and Application (CiRA), led by Dr. Yamanaka, some researchers are working on projects using iPS cells for drug discovery in monogenic and rare diseases. Research also is being conducted around the world on the use of iPS cells for regenerative medicine in sickle cell anemia, Parkinson’s disease, hemophilia A, spinal cord injury, and Fanconi anemia. The use of patient-specific iPS cells in regenerative medicine eliminates the ethical issues and immune rejection associated with ES cells. The caveat is that iPS cell generation and therapy is time- and money-consuming. Dr. Yamanaka has proposed creation of an iPS cell bank using iPS cells from donors. Finding the best cell type source and the best method for generating clinical-grade iPS cells is crucial to build a cell bank. Rigorous quality control is necessary for this project. Regarding the choice of donors, 50 unique HLA homozygotes (A, B, DR) would cover about 90% of the Japanese population, according to a study. Future research is needed to determine the best methods for differentiating iPS cells into functional cells, how to recapitulate pathology, how to transplant the cells, and how to address teratoma-related safety issues. Reprogramming-related issues are a unique concern because reprogramming is comparable to the pathogenesis of cancer. |