 |
|
|
 |
IS022
Use
of Gene Transfer as a Systemic Approach to Atherosclerosis |
|
Daniel J. Rader, M.D.
Department of Medicine
University of Pennsylvania School of Medicine
Philadelphia, PA, USA |
|
|
|
 |
|
|
 |
|
The current paradigm for acute coronary events is that
non-flow limiting lesions in the coronaries are often
the ones that rupture or fissure, generating thrombus
that causes acute coronary events. Interventions that
might cause regression or stabilization of lesions that
might cause acute coronary events have been sought. Proof-of
principle studies from Rader's laboratory in mice that
address some novel interventions that may be useful in
this manner were presented.
Interventions that target vectors to the liver may become
possible with demonstration of vectors that are safe,
able to target the liver and to express proteins at the
needed high levels. The liver would be used as a factory
to secrete circulating anti-atherogenic proteins with
the potential to interact with atherosclerotic lesions
systemically, or remain in the liver and modulate the
HDL reverse cholesterol transport pathway to reduce atherosclerosis
at distal vessel sites
|
PAGE
TOP
Role of HDL
and its metabolism |
|
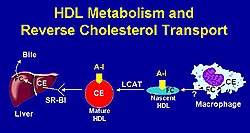 |
Figure
1. HDL metabolism involves a variety of gene products.
(FC, free cholesterol; CE, cholesterol ester;
A-1, apolipoprotein-1; HDL, high density lipoprotein;
LCAT, lecithin:cholesterol acyltransferase) (Rader
2000)
Click to
enlarge |
|
The epidemiologic observation that HDL cholesterol
is highly inversely associated with coronary risk
drove these studies. The Framingham Heart Study
has also shown that even when the LDL levels are
at acceptable levels, a very significant association
between risk and low HDL remained. Therefore, increasing
HDL levels might be a powerful modality to reduce
cardiovascular (CV) risk.
HDL metabolism is quite complex involving a
variety of gene products, and each is a potential
therapeutic target for atherosclerosis (Fig.1).
The ABC1 gene product facilitates removal of cholesterol
and phospholipids from cells. HDL and its major
protein, Apo A-1 transports cholesterol through
a series of steps ultimately resulting in the deposition
of the cholesterol ester in the liver by a cell
surface receptor, SR-B1, with excretion into the
bile. Conceptually, promotion of this pathway through
a variety of potential interventions could promote
removal of cholesterol from the vessel wall and
be a therapeutic intervention for atherosclerosis.
|
|
PAGE
TOP
|
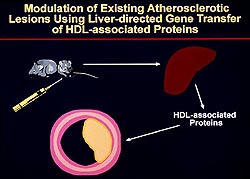 |
Figure
2. Study model of somatic gene transfer to the
liver to overexpress proteins when atherosclerotic
lesions had been induced in the animals. (Rader
2000)
Click to
enlarge |
|
Somatic gene transfer to the liver as a way of
overexpressing proteins at a time when atherosclerotic
lesions had been induced in the animals was used
(Fig. 2). Their studies were designed to study the
impact of gene overexpression in terms of regression
of atherosclerosis or changes in lesion morphology
when atherosclerosis was already present. This is
in contrast to transgenic mice models used to determine
which overexpressed gene prevent and reduce the
development of atherosclerosis.
A second-generation replication defective adenovirus
with an E1 deletion and a mutation of the E2a
gene, making the vector somewhat less responsive
to the E1-like cellular factors found in many cells,
particularly the liver, was used. A somewhat reduced
late gene transcription and therefore reduced immunoresponse
to the vector results. Expression is prolonged to
about 12 weeks in mice, allowing enough time to
look at atherosclerosis.
|
|
PAGE
TOP
HDL-associated
apolipoprotein studies |
|
|
|
ApoA-1 studies
To test
whether expression of HDL-associated proteins might
induce regression or changes in lesion morphology,
LDL receptor- (LDLR) deficient mice (n=38) were
fed a high fat, Western-type diet for 5 weeks which
induces fatty-streak lesions, not advanced lesions.
Of these 38 mice, 15 were assessed at baseline for
the extent of atherosclerosis, 12 were injected
with a second-generation adenovirus expressing human
ApoA-1, and 11 were injected with an adenovirus
not encoded with transgene. After 4 weeks quantitation
of the aortic atherosclerosis and cellular composition
of the atherosclerotic lesions were analyzed.
Significant regression of pre-existing atherosclerotic
lesions was found at 4 weeks in LDLR knockout mice
to less than a 1% aortic lesion from nearly 3% at
baseline, while the lesion continued to progress
in the adenovirus null group to nearly 4%. Further,
morphological changes were seen, with the lesions
in the ApoA-1 injected mice being smaller, flatter,
and less rich with foam cells. This is proof-of-principle
that even short-term expression of this major HDL
protein was able to induce fairly substantial regression.
|
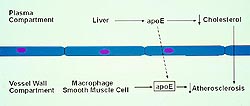 |
Figure
3. ApoE occurs in the plasma compartment and the
vessel wall compartment. (Rader 2000)
Click to
enlarge |
|
ApoE studies
ApoE is associated
with HDL and other lipoproteins, and is anti-atherogenic
(Fig. 3). Plasma-derived ApoE, mostly derived from
the liver, is known to have major effects on lipoprotein
metabolism. Vessel wall-derived ApoE is derived
from macrophages, both of which have been shown
to have has direct effects on atherosclerosis, including
progression.
Could the liver be targeted with an adenovirus
to cause ApoE expression into the blood, then the
plasma ApoE access the vessel wall and contribute
to the vessel wall pool of ApoE, and thereby induce
regression and morphologic changes?
ApoE expressed in ApoE knockout mice results
in a very profound reduction in plasma cholesterol
levels. In 12-week old ApoE knockout mice with early
fatty streak-type lesions at baseline, nearly complete
regression of lesions was found, whereas the lesions
progressed in the control mice. In 26-week old ApoE
knockout mice with more advanced lesions substantial
regression was seen, while lesions progressed in
the control mice. In the ApoE knockout mice, the
regressed lesions were flatter and more fibrous,and
the foam cells appeared to be very disrupted. Fibrous-type
caps also appeared to increase significantly, consistent
with the concept that lesions altered in this manner
results in more stable lesions.
Expression of ApoE in the liver only for 8
weeks resulted in a large amount of ApoE within
the lesions. Therefore, the effect was not due to
the adenovirus directly targeting the lesion of
the vessel wall. They believe the ApoE came from
the liver, directly targeted the lesions and was
retained by the lesions. So, this approach might
be useful to target atherosclerosis even when the
cholesterol levels are not being reduced.
ApoE in LDLR mice
Data from pilot
studies suggested that cholesterol levels were not
lowered with overexpression of ApoE in high-fat
diet, LDLR-deficient mice. In an experiment directed
at advanced atherosclerotic lesions, LDLR-deficient
mice (6 months of age) were fed a high fat, Western-type
diet for 12 weeks. A baseline group was assessed
for extent of atherosclerosis. The remaining mice
were injected with either human ApoE3 expressing
a second-generation adenoviral vector or the control
vector.
Little change in the plasma ApoE levels during
the 6-week study was seen in the control mice. In
the ApoE injected mice, a significant increase in
plasma ApoE to about 40 mg/dL at 5 days, compared
to about 10 mg/dL in the control, was seen. This
peak dropped off over time due to the immunoresponse
to the vector. But even at 6 weeks, the plasma ApoE
levels in the ApoE injected mice was twice that
in control mice (about 20 mg/dL vs 10 mg/dL, respectively).
Changes in cholesterol can not explain the
changes in atherosclerosis, as no difference in
plasma cholesterol between the two groups was found.
The ApoE resulted in substantial regression of the
lesion in the thoracic and abdominal aorta and some
in the aortic arch. In the baseline mice, the lesion
area was about 15%, in the control mice about 18%,
and in the ApoE mice about 5% (p=0.0001).
|
|
PAGE
TOP
|
ApoE has been reported to be an anti-inflammatory
and anti-oxidant protein in vitro. To test this
in vivo, isoprostanes were measured in the mice.
Isoprostanes 1) are produced by free radical catalyzed
peroxidation of fatty acids, 2) formed in vivo,
certainly in plasma and excreted in the urine, 3)
levels specifically reflect in vivo oxidative stress.
The control mice showed no change in isoprostanes
over the 6-week period. The ApoE-injected mice had
a dramatic reduction in isoprostanes, from 7.0 ng/mg
LDL at day zero, to 2.5 ng/mg LDL at day 7, to just
above zero at day 14. This reduction was maintained
for the 6 weeks. This is consistent with the possibility
that ApoE expression has an anti-inflammatory or
anti-oxidant effect that may have contributed to
its anti-atherogenic effects.
A pilot study using the AAV vector was performed,
as adenovirus vectors will not be used in human
studies. A recombinant AAV encoding the human ApoE3
gene yielded a fairly long-term expression of ApoE
in a dose-dependent manner. However, the plasma
levels of ApoE are substantially lower than those
achieved with adenovirus. Much work remains to refine
a vector that is less inflammatory and will express
for a long enough period of time.
|
|
PAGE
TOP
Cellular
proteins and HDL metabolism |
|
SR-B1 is a cell surface protein that mediates ester
uptake into the liver and targets it for excretion
into the bile. A substantial reduction in atherosclerosis
was seen in SR-B1 injected LDLR-deficient mice fed
a Western diet compared to control, consistent with
the concept that overexpression of SR-B1, despite
its reducing HDL, actually reduced atherosclerosis
perhaps by stimulating this pathway and reversing
cholesterol transport. LDLR-deficient mice with
established atherosclerotic lesions injected with
an adenoviral vector expressing SR-B1, which targets
the liver and remains there, had a dramatic 55%
reduction in the HDL peak with little effect on
VLDL and LDL at day 7. These results indicate that
HDL metabolism is affected by transient SR-B1 overexpression
even when VLDL and LDL are substantially elevated.
Further, it was found that atherosclerosis was significantly
reduced at two different time points by the transient
hepatic overexpression of SR-B1 in these LDL-R-deficient
mice. More studies are needed to determine how hepatic
SR-B1 overexpression is protective, but three potential
mechanisms may explain this effect. One, the expected
increased movement of HDL cholesterol through the
reverse cholesterol transport pathway may decrease
the rate of plaque deposition and may even have
effects on plaque regression. Two, HDL itself may
be altered and have increased antiatherogenic properties.
Three, non-HDL proteins may be changed in amount
or structure diminishing their atherogenecity. Based
on the analysis in this study of the lipoprotein
component levels and lesion size, it seems likely
that the changes in HDL cholesterol level had a
major impact on atherosclerosis. Their overall findings
suggest that interventions aimed at increasing hepatic
SR-B1 overexpression may be a novel approach to
the prevention and treatment of atherosclerosis,
since tissue distribution and regulation of SR-B1
expression are similar in mice, cultured human cells,
and humans.
|
|
PAGE
TOP
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2000
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|