|
The total length and the size of the AT1 and AT2
receptors are similar. The binding sites for these
two receptors are also similar. The third loop, which
is considered to be the major signal transmitter region
for both receptors, is the major difference between
the two receptors. However, the signal homology is
only about 33%. The AT2 receptor is the major focus
of this lecture.
Cloning of the AT1 and AT2 receptors from the bovine
and rat by this group showed a marked difference in
the sequence between the receptors in the area related
to the internalization of the receptor.
Two types of Ang II receptors exist: dithiothreitol-sensitive,
and dithiothreitol-insensitive. The AT1 receptor is
a specific AT1 blocker (losartan, candesartan, etc),
and the AT2 receptor is a specific AT 2 blocker (PD123319,
PD123177). The cloning and sequencing of these receptors
confirms the differences between these two receptors.
AT1 has a unique structure, while cloning showed that
the AT2 receptor has 34% amino acid identity with
AT1. However, the coupling of these receptors remains
controversial. Whether the AT2 receptor is always
Gi coupled and the AT1 receptor mainly Gq coupled
is unsettled.
The physiological function and regulation of the
AT1 and AT2 receptor differs, but further work must
be done to fully elucidate this matter. The AT2 receptor
is a growth inhibitor.
The regulation of AT2 is very complicated because
the expression of AT2 in the fetus is very high, and
it is mainly in the mesenchymal tissues. However,
immediately after birth, AT2 is reduced precipitously
and yet it remains at a finite level in certain tissues.
Data obtained from the vasculature cannot be transferred
directly to the heart. The heart seems to be more
susceptible to environmental changes. Both AT1 and
AT2 are expressed in cardiac fibroblasts and mycocytes.
AT2 in the myocytes is markedly reduced after birth,
but it is re-expressed under pressure overload and
in the failing heart, and probably Ang II is increased.
There is an intracardiac Ang II generating system
in cardiomyocytes, which is markedly stimulated by
myocyte stretch.
Inotropic and chronotropic actions are the short-term
effects of Ang II on the heart. The long-terms effects
include ventricular hypertrophy and dilated cardiomyopathy,
coronary artery remodeling, and cardiac ischemia.
AT1 is known to stimulate cellular growth. Many of
the growth mechanisms have been published, but it
remains unclear how these growth mechanisms are interrelated
and how these are related to AT2- stimulated cell
growth.
AT1 and AT2 are generally thought to work in opposite
directions. For example, AT1 stimulates cell growth
of fibroblasts, pheochromocytoma and neuroblastoma.
AT2 seems to suppress cell growth. In vivo,
the renal interstitial fluid production of nitric
oxide is stimulated by AT2, whereas it is not by AT1.
In the resistance vessels, AT-1 stimulates vasocontraction
and AT-2 stimulates vasorelaxation. Cell growth suppression
by Ang II via AT2 is done through the activation of
SHP-1, MKP-1, PP2A, and B2-NOS, among other factors.
|
|
Reported
Effects of AT2 on Cardiac Hypertrophy |
|
Modulation of the AT2 receptor has been done in various
ways by different investigators, such as AT deletion,
AT overexpression using transgenic technology, or
AT antagonism. This has yielded different results
regarding myocyte growth by AT, some showing it enhances
growth, some showing it suppresses growth, and some
showing no effect. The different results may be due
to the different techniques used, with newer techniques
being more accurate. Also, in terms of vascular function,
different results have been obtained by different
investigators regarding the effect of AT on vascular
smooth muscle cell (VSMC) hypertrophy. Again, different
methodologies may give rise to different results.
However, AT1 and AT2 also have parallel actions.
Aortic hypertrophy by Ang II in the rat aorta is blocked
by PD123319 rather than by losartan. Tyrosine hydroxylase
is stimulated by AT and AT2. Cardiac hypertrophy is
blocked by an AT1 or an AT2 blocker.
Inagami and colleagues showed using the AT2 knockout
left ventricular hypertrophy (LVH) model that aortic
banding and infusion of Ang II did not cause LVH in
the mice lacking the AT2 gene. Also, that the protein
level of p70S6k was significantly decreased in the
AT2 null mouse heart. All protein levels of the PI3
kinase-Akt/PKB-p70S6k pathway were decreased in the
AT2 null mouse heart.
Systolic blood pressure is elevated in the AT2 knockout
mouse, compared to the wild-type mouse. Interestingly,
aortic banding or angiotensin infusion increases myocardial
wall thickness in the wild-type mice, but in the knockout
mice, posterior wall thickness does not increase.
However, in terms of hemodynamic function, there was
no difference between the wild-type and the knockout
mice for LVDd and fractional shortening.
Wall thickness or cross sectional areas are determined
by PI3kinase, as shown by a number of studies. Protein
levels of the PI3kinase pathway were significantly
decreased in the AT2 null mouse heart with aortic
banding, whereas they are increased in the aortic-banded
wild-type mouse heart. Also, the heart size increases
in the wild-type mouse, to about the size seen in
cardiomyopathy, compared to the AT2 null mouse. The
myocyte cross-sectional area is increased about 2-fold
in the wild-type mouse, but there is little change
in the AT2 null mouse. Surprisingly, even collagen
type 1 synthesis increases in the aortic-banded wild
-type mice, but is inhibited in the aortic-banded
AT2 knockout mice.
In the AT2 null mouse heart, E/A is maintained without
a hypertrophic response with a 2-week infusion of
Ang II, compared to the wild-type mice. In contrast,
work by Harada showed that in AT1 knockout mice, Ang
II does not change the cross sectional areas. Also,
AT1 is a major factor in determining normal blood
pressure. A drop of about 35 mm Hg was seen in the
AT1 null mice. Interestingly, in the AT1 knockout
mice, aortic banding itself increased the cross-sectional
area. The increase was the same whether or not AT1a
is present. Fibrosis around the perivascular area
is present.
In sum, there are 2 major subtypes of the Ang II
receptor, AT1 and AT2. Traditionally, AT2 receptor
appear to have an antagonistic function against AT1.
However, the function of AT2 remains controversial.
Inagami and colleagues reported that pressure overload
or Ang II stimulation did not induce LVH in AT2 knockout
mice. However, recent studies reported that pressure
overload still induced LVH in AT1a knockout mice.
|
PAGE
TOP
|
Novel Signaling
Mechanism for AT2 Growth Promotion |
|
Inagami and colleagues examined the binding protein
through various segments of AT2 using the yeast two-hybrid
screening system, by using the AT2 carboxyl terminal
(C tail) as a bait to look for the DNA or protein
that binds the C-tail protein. They identified what
they named AT2CZ. Although AT2 C-tail has a binding
protein, it did not bind to the third loop. AT2CZ
is a specific binding protein, only binding to the
AT2 carboxyl terminus. The specificity of this binding
was confirmed using an in vitro binding assay.
Localization of AT2CZ shows its expression is tissue
selective. In the heart it is expressed in a large
amount, but it is not expressed in the large aorta.
It is expressed in a small amount in brain tissue,
in the colon it expresses a fairly large amount, perhaps
due to a different method being used for testing,
and in the kidney it is expressed in a limited amount.
Fortunately, the immortalized cell H9C2 expresses
AT2CZ, so perhaps this type of cell may be used more
extensively.
AT2CZ translocates to the nucleus by Ang II stimulation
via the AT2 receptor, but not by the AT1 receptor.
AT2CZ is localized on the periphery of the nucleus
after translocation.
AT2CZ does not seem to localize in any membranous
structure, even with Ang II present. When both AT2
and AT2CZ are present, Ang II stimulation results
in AT2CZ appearing in the perinuclear area within
about 30 minutes and begins to appear in the nucleus
in about 2 hours. In about 3 hours, AT2CZ is inside
the nucleus while AT2 remains on the periphery of
the nucleus.
Arrestin is not involved in the AT2-AT2CZ complex.
Arrestin also binds the C tails of various G-protein
coupled receptors, including AT1. So, using AT1-expressing
cells and beta-2 arrestin-expressing cells, Inagami
and colleagues showed there is binding about 15 minutes
after stimulation by Ang II. In contrast, AT2-expressing
cells do not bind in response to beta-2 arrestin.
So, this is a rare receptor not bound by beta arrestin.
Interestingly, Ang II induces AT2 internalization
in the cell membrane in the presence of AT2CZ. In
contrast, if AT2CZ is present, Ang II stimulation
results in a reduced amount of AT2 receptors on the
cell membrane. This may indicate that AT2 has begun
to be internalized into the cytosoles and nucleus.
In the nucleus, the DNA element in the PI3K 85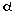 5”-flanking
region binds to the AT2CZ zinc finger domain. This
was shown by Inagami and colleagues in a consensus
sequence in which various mutations were introduced.
Ang II induces P13K 85 5”-flanking
region binds to the AT2CZ zinc finger domain. This
was shown by Inagami and colleagues in a consensus
sequence in which various mutations were introduced.
Ang II induces P13K 85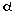 expression
mediated by AT2 in the presence of AT2CZ. Also, in
R3T3 cells, Ang II greatly increases P13K 85 expression
mediated by AT2 in the presence of AT2CZ. Also, in
R3T3 cells, Ang II greatly increases P13K 85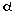 expression.
They also showed that in the cells containing the
CZ factor that AT2 stimulates the nucleus to express
PI3K. Shioi et al showed that this is the mechanism
that activates many of the cardiac hypertrophic factors,
including calcineurin. Thus, Inagami and colleagues
think this is one of the mechanisms whereby AT2 induces
cardiac hypertrophy. The change in the PI3K pathway
is time dependent, as a consequence of internalizing
the CZ gene. expression.
They also showed that in the cells containing the
CZ factor that AT2 stimulates the nucleus to express
PI3K. Shioi et al showed that this is the mechanism
that activates many of the cardiac hypertrophic factors,
including calcineurin. Thus, Inagami and colleagues
think this is one of the mechanisms whereby AT2 induces
cardiac hypertrophy. The change in the PI3K pathway
is time dependent, as a consequence of internalizing
the CZ gene.
Ang II increases leucine uptake via the AT2-AT2CZ
complex, indicating a hypertrophic response. Leucine
uptake is inhibited by PD compounds, but not by losartan,
indicating a hypertrophic response.
|
PAGE
TOP
|
|
Inagami and colleagues identified a new zinc finger
protein, AT2CZ, which binds AT2. AT2CZ translocates
to the nucleus by Ang II stimulation via AT2, but
not AT1. AT2CZ increases P13K 85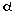 expression level 1 hour after Ang II stimulation.
AT2 may work as a growth promoter in the presence
of AT2CZ. If both AT1 and AT2 receptors have hypertrophic
effects, an ACE inhibitor is a better choice than
an AT1-specific receptor blocker to prevent or treat
cardiac hypertrophy. CZ was discovered as a switch
for hypertrophic or growth inhibitory mechanisms by
AT2. CZ and its related signaling mechanism provide
a target for cell growth control.
expression level 1 hour after Ang II stimulation.
AT2 may work as a growth promoter in the presence
of AT2CZ. If both AT1 and AT2 receptors have hypertrophic
effects, an ACE inhibitor is a better choice than
an AT1-specific receptor blocker to prevent or treat
cardiac hypertrophy. CZ was discovered as a switch
for hypertrophic or growth inhibitory mechanisms by
AT2. CZ and its related signaling mechanism provide
a target for cell growth control.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2003
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|