 |
|
|
 |
| New Horizons in the Management of Patients with Chronic Heart Failure |
|
|
|
 |
|
Overview of ACC/AHA Guidelines for Chronic Heart Failure
Sharon A. Hunt
Stanford University, Stanford,
CA
|
|
Heart failure is
a major public health issue, with 5 million people
in the US and an
estimated 22 million worldwide with heart failure.
The annual death rate for heart failure is 300,000.
Heart failure is the only cardiovascular (CV) diagnosis
that is increasing. An enormous population of older
people with heart failure is being created, because
of the aging of the population and increasingly effective
therapies that prolong life for people with heart
failure. Sudden cardiac death is also being prevented
in these patients, increasing the size of the population
requiring management.
A major message
from the guidelines is that there is a large body
of evidence showing that heart failure can be prevented
or at least delayed, by a number of modalities. In
the US and worldwide,
what is known to be effective is underutilized. A
plea for the more effective use of the evidence base
is a major thrust of the updated guidelines.
The ACC/AHA guidelines
address only chronic heart failure and heart failure
in the adult. The treatment of acute heart failure
merits its own set of guidelines. All of the major
trials and hence the evidence-base is in adults and
there is little real evidence-base in the pediatric
population. In general, the causes of pediatric heart
failure are different.
Stages of heart failure
Heart failure is
viewed as a set of stages. Stage A is persons
at high risk for the development of heart failure.
This is to promote awareness of the large number of
people at high risk and effective therapies to prevent
heart failure. Stage B is persons with asymptomatic
left ventricular dysfunction (ASVD), and who are usually
discovered to have this condition and are at risk
for clinical heart failure. Stage C is persons
with past or current symptomatic heart failure. Stage
D is persons with end-stage heart failure who
are dying from heart failure.
Importantly, the
four stages in the ACC/AHA guidelines do not replace
the NYHA classes of heart failure, but are intended
to complement the guidelines. The stages
progress inexorably from Stage A to Stage D, whereas
movement within the NYHA classes can be in both directions. The stages are specifically
designed to emphasize that heart failure is preventable,
with emphasis on identification and treatment to prevent
heart failure.
Therapy by stages
Stage A
therapy consists of treatment of the specific risk
factors in a patients, such as hypertension, diabetes,
dyslipidemia, ASVD, and controlling conditions that
may cause cardiac injury.
Stage B
therapy is the treatment of risk factors and the use
of ACE inhibitors to delay the onset of heart failure
and improve survival. Whether the addition of beta
blockers in this asymptomatic group will have similar
salutory effects is not known as it has never been
studied in a clinical trial. The consensus, despite
the lack of evidence, is that beta blockers are a
reasonable addition and they probably should be given
as tolerated to patients with ASVD.
Active screening
of the population for ASVD has not been the subject
of a clinical trial and is unresolved. Although the
panel consensus is that active screening would be
productive, there is no consensus within the profession
on the advisability of such an expenditure.
Screening would probably need to be restricted to
a high-risk population because of expense.
Stage C
outlines the evidence-based therapies. The treatment
of underlying risk factors and other preventive measures
should be undertaken. Drugs to avoid in these patients
include most antiarrhythmic agents with the notable
exception of amiodarone, most calcium antagonists
with the exceptions of amlodipine and felodipine,
and NSAIDs, which can exacerbate the clinical syndrome.
Multiple drug therapies
are required. However, many of these modalities are
grossly underutilized, perhaps in part because of
their complexity. Drugs recommended for routine use
in virtually all patients are the █gbig four█h: diuretics,
ACE inhibitors, beta blockers, digitalis. Importantly, practitioners should use the specific
agents that have been proven in the
clinical trials, and not assume class effects for
all drugs, and they should aim for the actual
doses proven to be effective in the trials.
Drugs that should be considered in subsets of patients
with heart failure include the 1) aldosterone antagonists,
2) angiotensin receptor blockers as alternatives to
ACE inhibitors in specific patients, 3) the combination
of hydralazine and nitrates, and 4) exercise training,
which has been shown to improve quality of life.
Drugs and interventions
of unproven benefit, that are popular in the therapy
of heart failure, include nutritional supplements,
hormonal therapies, intermittent intravenous inotropes,
and cardiomyoplasty, which is
used little now.
Stage D
therapy includes all therapies used for Stages A through
C. For patients who are truly end-stage, practitioners
often face the difficult decision of whether to identify
them as part of group of patients who should receive
aggressive therapy and offer mechanical and surgical
strategies, or attempt to provide compassionate end-of-life
care. For the vast majority of patients dying of heart
failure, compassionate end-of-life care is the most
appropriate and is often not carried out well by cardiologists,
and much can be learned from oncologists.
Cardiac transplantation
is the most tried and true aggressive intervention.
It provides an excellent survival rate and quality
of life, but quantitatively is a small contribution
to the number of deaths from heart failure. Much is
written and discussed about high-risk surgery for
these patients, include mitral valve repair, surgical
remodeling of left ventricle, and most recently on
the horizon is the potential use of permanent mechanical
circulatory support.
|
PAGE
TOP
|
Beta-Adrenergic
Receptor Blockade in CHF: A Rational Tool for the
21st Century
Tsutomu Yoshikawa
Keio University School of Medicine,
Tokyo, Japan
|
|
Congestive heart
failure is characterized by activation of the sympathoadrenal
and renin-angiotensin systems. The sympathoadrenal
system is essential to maintain the circulatory system
in the face of stress. However, activation of this
system over time has been shown to adversely effect
the pathophysiology via multiple mechanisms, such
as cardiac hypertrophy, oxygen free radicals, proinflammatory
cytokines, matrix matalloproteinase, in addition to
the classic concept of energy expenditure through
increased oxygen consumption.
The adverse effect
of protracted sympathoadrenal activation was recently
shown by data from transgenic mice overexpressing
beta-1 adrenergic receptors. The mice initially exhibited
hypercontractility, but eventually cardiac hypertrophy
and failure, resulting in premature death.
Large-scale clinical
trials with carvedilol, bisoprolol, and metoprolol
have demonstrated that beta-blockers (BB) improved
survival in patients with mild to moderate heart failure.
|
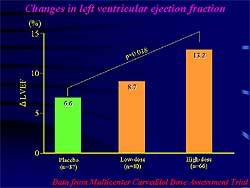 |
| Figure
1. Carvedilol dose-dependently increases echo-defined
left ventricular ejection fraction. |
| Click
to enlarge |
|
Carvedilol was shown
effective in advanced heart failure in the COPERNICUS
trial, and it reduced total mortality by 65% in the
US Carvedilol Heart Failure Study. There are few data
on clinical trials in the Japanese heart failure population.
Yoshikawa and colleagues showed in a randomized, placebo-controlled
clinical trial that carvedilol significantly reduced
CV events compared to placebo. Notably, even low dose
carvedilol (5 mg daily) was beneficial in preventing
CV events in this population.
However, echocardiographic
left ventricular ejection fraction (LVEF) was higher
in the high-dose versus the low-dose group at the
end of study, showing a dose-response to carvedilol
for left ventricular function (Figure
1). The MOCHA trial demonstrated a dose-dependent
decrease in mortality with carvedilol. Hence, BB should
be uptitrated to the maximum dose, if tolerated, although
low-dose BB are useful in preventing CV events.
|
|
Are there differences between the
first-generation BB such as metoprolol and third-generation
BB such as carvedilol? Yoshikawa and colleagues propose
that agonism and the antiadrenergic effect are the
critical pharmacologic profile in this regard. BB
have been thought to compete with intrinsic norepinephrine
against uniform beta-adrenergic receptors. However,
recent findings from transgenic mice suggested there
are 2 forms of receptors, active and inactive in the
absence of endogenous norepinephrine, which is referred
to as █ginverse agonism.█h Because BB with such inverse
agonism transforms active receptors to inactive receptors,
in the milieu of low sympathetic activity, adverse
cardiac events including worsening heart failure will
occur during the titration period.
These investigators
showed that metoprolol dose-dependently decreased
adenyl cyclase activity in the absence of endogenous
norepinephrine, suggesting inverse agonism, in a hamster kidney cell line transfected
with baculovirus encoding human beta-1 adrenergic
receptors. A separate comparison of the magnitude
of inverse agonism among different BB by this group
showed that it was prominent in metoprolol, minimal
in bucindolol, and moderate in carvedilol (inhibition
of adenyl cyclase activity 60%, 38%, and 55%, respectively).
The clinical significance
of inverse agonism in heart failure patients has not
been shown. Gong and colleagues
showed that the inverse agonist ICI 118,551 had minimal
inverse agonism on the contraction of isolated non-failing
cardiomyocytes, but had profound inverse agonism in
failing myocytes, showing this pharmacologic profile
is important in heart failure. Bristow and colleagues
showed that propranolol and metoprolol with inverse
agonism decreased cardiac output and increased pulmonary
capillary wedge pressure. Carvedilol and bucindolol
with modest inverse agonism coupled with vasodilatory
activity were associated with minimal hemodynamic
deterioration and decreased pulmonary capillary wedge
pressure.
In the MERIT-HF
trial, metoprolol decreased adverse cardiac effects
during the titration period compared to placebo. However,
in moderate heart failure, adverse cardiac events
were increased with metoprolol during the titration
period. In contrast, in more advanced heart failure,
the COPERNICUS study showed no such trend. The withdrawal
rate from the study was essentially the same between
carvedilol and placebo. This different finding may
be explained by the difference in inverse agonism
between carvedilol and metoprolol.
Anti-adrenergic effect
The effect of the
BB on the density of beta-adrenergic receptors is
another critical pharmacologic property. This group
showed that the number of receptors markedly increased
with metoprolol, but decreased with carvedilol in
chick heart cells expressing predominantly beta-1
adrenergic receptors. This suggests that carvedilol
may exert a more potent anti-adrenergic effect than
metoprolol.
Work by Gilbert
and colleagues showed that metoprolol increased and
carvedilol decreased the number of receptors in a
biopsy sample from the right ventricle. Coronary sinus
norepinephrine concentration was significantly decreased
by carvedilol but not by metoprolol. In this regard,
carvedilol exerts a more potent anti-adrenergic effect
than metoprolol, because carvedilol does not elicit
upregulation of beta-adrenergic receptors.
|
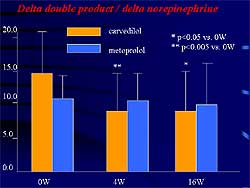 |
| Figure
2. Carvedilol but not metoprolol significantly
decreased the delta double product during maximal
exercise in patients with mild to moderate heart
failure. |
| Click
to enlarge |
|
This group showed
in patients with mild to moderate heart failure that
the increase of delta double product during maximal
exercise is normalized by an increase in plasma norepinephrine
concentration, which is a parameter for adrenergic
responsiveness (Figure
2). Carvedilol but not metoprolol significantly
decreased this parameter during the study, suggesting
that carvedilol exerts a more potent antiadrenergic
effect than metoprolol during exercise.
The magnitude of
increase in LVEF was larger with carvedilol than with
metoprolol in the largest study to compare the two
drugs. In contrast, maximal oxygen consumption during
exercise was significantly increased with metoprolol
but not by carvedilol. These results may reflect the
difference in antiadrenergic effect during stress
between the two drugs.
|
|
Idiopathic cardiomyopathy (IDCM)
is a relatively common cause of heart failure in Japan.
About 40% of such patients have autoantibodies directed
against beta-1 adrenergic receptors. Immunoglobin
G fraction isolated from rabbit serum immunized by
the second extracellular loop of beta-1 adrenergic
receptors, dose-dependently increased adenyl cyclase
activity in rabbit cardiac membrane preparation, indicating
that this autoantibody has an agonist-like effect.
Bispoprolol inhibited adenyl cyclase activity in the
absence of IgG or endogenous norepinephrine, suggesting
inverse agonism in a study by this group. Bisoprolol
completely abolished the agonist-like action of the
autoantibodies.
To determine the
clinical significance of the autoantibody directed
against beta-1 adrenergic receptors, Yoshikawa and
colleagues studied 104 patients with IDCM. The presence
of the autoantibody was associated with high-risk
ventricular tachycardia on Holter monitoring. There
was no difference for heart failure death between
patients with and without autoantibodies, but sudden
cardiac death was significantly higher in patients
with the autoantibody than those without (p=0.0219.
Cox proportional hazards analysis revealed that the
presence of the autoantibody was an independent predictor
for sudden cardiac death and low EF. BB use was a
negative predictor for high-risk ventricular tachycardia
in patients with autoantibodies. This finding suggests
that BB are useful to prevent sudden cardiac death
(SCD) from serious ventricular arrhythmias in autoantibody-positive
with IDCM.
In summary, significant
differences in the pharmacologic profile of metoprolol
and carvedilol exist. Inverse agonism, an agonist-independent
receptor inactivation, is more prominent with metoprolol
than carvedilol. This pharmacologic property may be
relevant to adverse cardiac events during the introduction
of beta blocker therapy in severe heart failure. Metoprolol,
but not carvedilol, caused up-regulation of beta-adrenergic
receptors. Carvedilol decreased the number of receptors
in an in vitro experiment. In patients with
mild to moderate heart failure, carvedilol exerts
a more potent antiadrenergic effect during exercise
than metoprolol. The presence of an autoantibody directed
against beta-1 adrenergic receptors are associated
with SCD, related to serious ventricular arrhythmias
in patients with IDCM. The presence of inverse agonism
seems to be an advantage in patients with mild heart
failure from IDCM with autoantibodies directed against
beta-adrenergic receptors.
|
PAGE
TOP
|
The
Role of Angiotensin Converting Enzyme Inhibitors (ACE-I),
Angiotensin II Type I Receptor Antagonist (ARBs) and
Aldosterone Receptor Blockers (AB) in Chronic Heart
Failure (HF)
Bertram Pitt
University of Michigan School
of Medicine, Ann Arbor, Michigan
|
|
The RALES study
randomized patients with severe heart failure to Aldactone™
spironolactone and placebo, and showed a 30% reduction
in all-cause mortality, from a reduction in sudden
cardiac death (SCD) and progressive heart failure.
This strategy in severe heart failure is being adopted
in many parts of the world.
Spironolactone is
known to cause gynecomastia and breast pain in men
(about 10% of men in RALES) and menstrual irregularities
in women. Although this may not be a major defect
in patients with severe heart failure, this prohibits
the application of this strategy in less severely
ill patients, patients with mild to moderate heart
failure, asymptomatic left ventricular dysfunction,
and hypertension.
A new aldosterone
blocking agent, eplerenone, is similar in its action
to spironolactone, but it is more specific for the
aldosterone receptor and does not bind to androgen
and prostogen receptors and does not appear to cause
gynecomastia, breast pain, and menstrual irregularities.
This new compound may have wider applicability and
hence intensive study has been underway.
In the EPHESUS study, eplerenone
was studied in patients with AMI with systolic dysfunction
(LVEF ≤ 40) and rales in non-diabetic patients.
Patients were treated with standard therapy and randomized
to eplerenone (n=3,100) starting at 25 mg daily for
1 month and uptitrated to 50 mg daily or to placebo
(n=3,100). Patients were randomized between 3 and
14 days post-MI, the mean time was 7.3 days. The trial
was carried out until it reached 1,012 deaths. The
two primary endpoints were all-cause mortality and
the combination of cardiovascular mortality and CV
hospitalizations (MI, stroke, heart failure, ventricular
arrhythmias). In contrast to the RALES study in which
only 10.5% of patients were on beta blockade, 74%
of EPHESUS patients were receiving beta blockers.
Mechanisms for efficacy of
angiotensin blockade
The RALES trial
showed that the diuretic effect of aldosterone blockade
did not appear to be the major mechanism for reducing
mortality. In RALES patients with class IV heart failure
had a higher sodium retention score than patients
in class III, but there was no difference between
the patients randomized to spironolactone or placebo,
suggesting that although there may have been some
diuretic effect of spironolactone it did not appear
to account for the major benefit.
Aldosterone plays
a critical role in many other neurohumoral pathways.
There are a number of vicious cycles. For example,
aldosterone upregulates tissue ACE activity and thereby
causes more angiotensin II which causes more aldosterone.
Another cycle is with endothelin-1, affects on norepinephrine,
and vasopressin. Many of these neurohormones combine,
especially aldosterone, to increase NADH/NADPH oxidases
in the vascular wall, cause the release of free radicals,
and thereby stimulating various signaling pathways,
such as NF-κB and AP-1 and destroy nitric oxide (NO).
In the lipid-fed
rabbit model, a marked increase in vascular NADH/NADPH
was seen compared to normal control rabbit with saline
in work by Pitt and colleagues. Eplerenone nearly
eliminated the NADH/NADPH activity. Free radical production
was markedly increased in this model because of the
stimulation of NADH/NADPH, but it was significantly
and markedly decreased with eplerenone. Normal animals
vasodilated whereas the lipid-fed model had endothelial
dysfunction in response to acetylcholine; eplerenone
caused significant improvement but not a total correction
of endothelial function.
Work by Bauersachs
and colleagues in the rat model compared the ACE inhibitor
trandolapril to spironolactone and showed that spironolactone
decreases NADH/NADPH and improves NO availability
by decreasing free radical formation, whereas the
ACE inhibitor trandolapril improved NO synthase. Combining
the drugs caused a marked improvement in NO and completely
corrected endothelial function in the animal models.
Farquharson and
colleagues randomized patients with mild to moderate
heart failure who had been on an ACE inhibitor for
3 months to spironolactone or placebo. Spironolactone
caused a significant improvement in endothelial function
in response to acetylcholine compared to placebo (170%
change vs 99% change in FBF, respectively). This may
be one important mechanism for aldosterone blockers
because patients with heart failure have a high incidence
of endothelial dysfunction and a high circulating
level of free radicals.
Work by Ganten and
Luft in the double transgenic mouse showed that aldosterone
is an important stimulator of the NF-κB and AP-1
signaling pathway. Aldosterone and angiotensin II
were compared in this model showing that angiotensin
II is perhaps a better stimulator of NF-κB whereas
aldosterone is a better stimulator of the AP-1 signaling
pathway. Both angiotensin II and aldosterone activate
both ways.
Work in an animal
model has shown that aldosterone causes a marked increase
in various adhesion molecules and cytokines. Cox-2,
osteopontin, and MCP-1 are upregulated. There is marked
microvascular inflammation, as a result of aldosterone
stimulation, causing subsequent fibrosis. Angiotensin
blockade can block all of these effects. A model of
aldosterone/salt hypertensive rats showed marked fibrosis,
which was significantly prevented with eplerenone.
This was also seen in a substudy of the RALES trial,
patients randomized to spironolactone had a marked
decrease in ongoing collagen formation, which seemed
to correlate with the improvement in survival. Suzuki
and co-workers showed in an animal model that eplerenone
decreases levels of MMP 2 and MMP 9 and thereby prevents
progressive left ventricular remodeling.
Aldosterone improved
heart rate variability, which may be a mechanism by
which it prevents sudden cardiac death. So, it restores
autonomic balance by improving vagal tone through
an increased availability of NO. Many studies have
shown that increased heart rate variability correlates
with a reduction in SCD.
Aldosterone prevents
the uptake of norepinephrine into the myocardium and
increases circulating catecholamine levels. Conversely,
aldosterone blockade improved myocardial norepinephrine
uptake and decreases circulating catecholamine levels.
Cytokines and TNF-alpha
are increased after myocardial damage. TNF-alpha increases
prostaglandin-E2, which can cross the blood-brain
barrier and stimulate central mineralocorticoid receptors.
Their activation causes a central increase in sympathetic
drive, salt appetite, blood volume, hypertension,
AVP, angiotensin II binding to the AT-1 receptor,
and a further increase in TNF-alpha. This is another
vicious cycle, and the activation of the central sympathetic
drive may be an important mechanism in SCD which can
be turned off by aldosterone-receptor blocking agents
which can cross the blood-brain barrier.
Another new concept
is that aldosterone blocking agents may be affected
when serum levels of aldosterone are not elevated.
One explanation may be that the mineralocorticoid
receptor can be occupied, but it can be occupied also
by cortisol. Normally cortisol is rapidly degraded
by the enzyme 11-ßHSBD2, and the cortisone cannot
occupy the receptor. So, normally all the aldosterone
occupies the receptor, however, during oxidative stress
that enzyme is down-regulated and there may be more
cortisol available and it may occupy the mineralocorticoid
receptor. An agent such as spironolactone or eplerenone
can block that receptor regardless of whether or not
it is occupied and activated by aldosterone or cortisol.
This may in part explain why in many of our studies
even where serum aldosterone levels are not elevated
there is a striking effect of aldosterone blockade.
The current concepts
of aldosterone blockade are: The mineralocorticoid
receptor can be stimulated by aldosterone or cortisol,
and this receptor can be blocked and the effects of
either aldosterone or cortisol on this receptor. This
receptor has a number of effects: the classic effects
on the kidney; increases vascular NADH/NADPH, leading
to an increase in free radicals; reduction in NO which
has many important adverse effects; increase of free
radicals; stimulation of NF-κB and AP-1, adhesion
molecules, vascular inflammation, fibrosis and hypertrophy.
All of these may lead to hypertension, heart failure,
and ischemic events. The mineralocorticoid receptor
may centrally and peripherally effect autonomic balance
and cause changes in heart rate variability, baroreceptor
function and norepinephrine uptake, which can lead
to sudden death. Blocking this receptor has a number
of beneficial effects, and may in part explain the
results of the RALES and EPHESUS trials.
|
PAGE
TOP
Disease Management for Chronic Heart Failure: Opportunity and Application
Harlan M. Krumholz
Yale University School of Medicine, New Haven, CT
|
|
The need for a disease
management strategy in heart failure is illustrated
by a typical heart failure patient. She is a 76-year-old
grandmother with diabetes, hypertension, arthritis,
and mild chronic obstructive pulmonary disease, and
heart failure. The medications for the different co-morbidities
can work at cross-purposes, perhaps helping one condition
but perhaps adding risk to another condition. Polypharmacy
also affects compliance and affordability of treatment,
among other factors.
Concerns at hospital
discharge include 1) inadequate explanation and understanding
of the medications, and 2) lack of optimal communication
between the hospital and the follow-up physicians,
adding to patient confusion about treatment regimen,
which can lead to hospital re-admission. A vicious
cycle is often set up. Despite the advances in the
treatment of heart failure, the medical system is
failing these patients. The patients are repeatedly
hospitalized and their quality of life (QOL) is compromised.
More global thinking about the cluster of conditions
of the patient and how to help the patient manage
them is required.
The conditions commonly
present in patients with heart failure are hypertension
60%, renal sufficiency 50%, and diabetes 33%, according
to a national survey in the US. Further,
the outcomes are 10% mortality rate at 30-days, 25%
at 6 months, and 35% at 1 year. The rate of hospitalization
is 1 in 5 patients at 30 days, 1 in 2 patients at
6 months, and 66% at 1 year.
The burden on health
care system is illustrated by a survey in the state
of Connecticut conducted
by Krumholz and colleagues. The all-cause hospital
re-admission for a patient with heart failure was
$7000. A low-risk or high-risk group for readmission
could not be identified.
Importantly, the
patient composition in clinical practice and clinical
trials differ. In clinical practice, 50% of patients
are women with an average age of 75 years, while only
20% of the trial population is women and the average
age is 60 years, according to a systematic review
of clinical heart failure trials by Krumholz and colleagues.
Underutilization
of appropriate testing and treatments is known. A
national survey showed that assessment of left ventricular
ejection fraction (LVEF) while hospitalized or documented
from a prior measurement occurred in only 60% of patients.
ACE inhibitors for LV systolic dysfunction were prescribed
for only 60% of patients. In some patients a therapeutic
substitution of an angiotensin receptor blocker for
an ACE inhibitor was made, although this is not supported
by the guideline.
Communications gaps
in patient care are affecting outcomes. This is seen
in the limited counseling at discharge about medications,
weight monitoring, diet, activity, follow-up appointments,
and management of symptoms, as shown in the national
survey. Systems must be developed to ensure discharge
communication occurs.
Gaps in treatment,
education, and continuity of care exist, as shown
by the national survey and other data. The health
care system is designed to react to illness, not to
be proactive and prepared to prevent these exacerbations.
Disease management for heart
failure
Patient management
includes 1) ensuring the patients has the best treatment,
and 2) has the information, skills and support to
make good decisions to manage their heart failure
in partnership with their physician and other
caregivers.
Disease management
provides support to patients and health care providers.
It ensures that patients receive needed treatment
and information. It combines a prepared patient and
family with a prepared health care system that seeks
to be proactive and prevent disease.
Krumholz and colleagues
studied the effect of an educational strategy on outcomes.
The educational strategy was
designed to provide the patient with information about
their illness, the relation between their medication
regimen and their health, the relation between their
health behaviors and their health, about early signs
of decompensation, and where and when to obtain assistance.
The education strategy included in-hospital counseling
and phone calls at intervals to reinforce the concepts.
In the educational strategy group, survival free of
heart failure readmission was 30%, all-cause readmission
or death 37%, cardiovascular readmission or death
40%, and heart failure readmission 43%. The hospital
costs were higher in the control group by an average
$7000; $25,000 vs $14,000 per year. The educational
strategy was cost saving.
A systematic review
of 17 studies of post-discharge support showed a remarkable
26% risk of readmission in heart failure patients.
The interventions included single-home visits, increased
clinic follow-up and telephone contact.
The generalizability
of these findings to other populations is needed.
Payment remains a problem. Despite the remarkable
results, no one is willing to pay for the preventive
intervention. Systems continue to focus on paying
for acute illness, not prevention. The value of these
approaches has not been integrated into the health
care system. To put this in perspective, a drug that
reduced readmission by 25% thus saving money and had
no side effects would find its way into the health
care system.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2003
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|

