|
The role of the immune system in atherosclerosis
and the potential for exploiting the immune system
to influence the atherosclerotic disease process are
new concepts in atherogenesis and atherosclerosis
management.
The primary goals of the Atherosclerosis Research
Center at Cedars-Sinai are: 1) to gain an understanding
of the molecular mechanisms of atherothrombosis, focusing
on novel genes, 2) to use this improved understanding
to develop and test novel therapies that can prevent,
reverse, or stabilize atherosclerosis. Some of the
novel genes identified at this research center through
transcriptional profiling of various arteries include
PTN, LPP, TNF-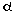 ,
and M-CSF. ,
and M-CSF.
HDL-based therapeutics, the development and introduction
of the recombinant apo A-1 milano, and oral apo A-1
limited peptide therapy are some of the novel therapies
for atherosclerosis studied at the research center.
Promising results with recombinant apo A-1 milano
are being seen, which is already in clinical trials;
much of the pre-clinical work was done at Cedars-Sinai.
Gene transfer, using novel vectors to introduce therapeutic
genes, is another program at the research center.
The benefits of a number of atheroprotective genes,
including Apo A-1 wild-type and Apo A-1 milano, is
being explored in animals, with the hope of subsequent
study in humans. The role of immune mechanisms and
developing novel ways of modulating atherosclerosis
by influencing the immune system constitutes another
large program at the research center.
|
|
The immune
system in atherosclerosis |
|
Inflammatory gene induction and immune cell activation
plays a critical role in every step of the evolution
from a normal artery to an artery with an atherosclerotic
lesion and subsequent plaque rupture and thrombosis.
The retention and modification of atherogenic lipoproteins
at atherosclerosis-prone sites activate the inflammatory
and immune mechanisms that lead to the earliest stages
and then progression of atherosclerosis and eventually
to de-stabilization of atherosclerosis.
The evidence for the involvement of the immune system
in atherosclerosis comes from animal and human models
of the disease: 1) In animal models, the presence
of activated T-cells, macrophages, mast cells, and
dendritic cells, all components of the innate and
adaptive immune response, can be found at the earlier
stages of lesion evolution. 2) T-cells reactive to
various antigens can be demonstrated in atherosclerotic
lesions and in circulating blood. 3) Antibodies to
a variety of auto-antigens are found in circulating
blood and atherosclerotic lesions. 4) T-cell-dependent
cytokines can influence and modulate atherosclerosis.
5) Immunization and tolerization experiments clearly
can modulate atherosclerosis in a favorable or unfavorable
direction. 6) Pro-atherogenic and anti-atherogenic
immune responses can be demonstrated to be transferable
using adaptive cell transfer techniques. All of these
data suggest that the immune system plays an important
role in modulating experimental atherosclerosis.
Similarly, in human models of atherosclerosis, there
is evidence for a role of the immune system. The presence
of all of the cells involved in the immune response
has been shown in the earliest to most advanced stages
of atherosclerotic lesions. As in animals, antibodies
and reactive T-cells can be demonstrated. Pro-atherogenic
effects of immune suppression have been documented
in transplant medicine in humans. Accelerated atherosclerosis
in autoimmune disease, such as lupus, is further evidence.
Atheroprotective effects of non-specific vaccines,
such as influenza vaccine, have recently been demonstrated
in cross-sectional and pilot clinical trials in humans.
|
PAGE
TOP
|
Innate immunity
and atherosclerosis |
|
The macrophage is the major factor in innate immunity.
It is a blank response of macrophages, with the receptors
CD-36, toll-like receptor (TLR), and SR-AB. When these
receptors are engaged by a variety of pathogen-associated
molecular patterns (PAMPS) and oxidized lipids, it
leads to either foam cell formation in the case of
the scavenger receptor or CD-36, or to activation
of inflammatory and immune active genes with the engagement
of TLR, and then the release of inflammatory mediators.
TLR-4 is a transmembrane receptor, which, along
with other co-receptors such as CD-14, is able to
signal the effects of bacterial lipopolysaccharide,
endotoxin, or heat shock proteins (HSP). Through adaptive
molecules, such as MD-88, it is able to activate and
translocate NF-ɻB to the nucleus, leading
to the induction of inflammatory and immune genes
and mediators.
TLR-4 immunoreactivity can be detected in lipid-rich
atherosclerotic plaques and co-localizes with macrophages,
as recently demonstrated by Shah and colleagues in
collaboration with another laboratory. TLR-4 is primarily
expressed by macrophages and by the endothelial cells
in human and murine atherosclerotic lesions. Interestingly,
TLR-4 mRNA is upregulated in a dose-dependent fashion
by oxidized LDL, but not by native LDL—indicating
a link between lipoproteins and the innate immune
system.
|
PAGE
TOP
|
TLR-mediated
signaling in murine atherosclerosis |
|
The innate immune system seems to play a pro-atherogenic
role. TLR signaling is responsible for linking some
of the pro-inflammatory effects of hyperlipidemia
in the murine model and thus plays an important role
in atherogenesis.
In experiments with double-knockout mice that lacked
the ApoE gene and either one or both alleles of the
MyD88 gene (myeloid differentiation factor), a critical
adapter protein in the signal transduction cascade
for TLR, Shah and colleagues showed that the introduction
of the MyD88 null genotype, in a dose-dependent fashion,
is associated with a reduction in atherosclerosis.
The double-knockout mice homozygous for the MyD88
knockout had about a 55% reduction in atherosclerosis.
In addition, a dose-dependent decrease in plaque inflammation
was measured by macrophage immunoreactivity, with
about a 60% reduction when both alleles of the MyD88
gene had been knocked out. Clearly, the innate immune
system is playing a pro-atherogenic role. Disrupting
the innate immune signaling through disruption of
the MyD88 gene results in a major reduction in atherosclerosis
and plaque inflammation.
Notably, the effects on atherosclerosis are not
mediated through a reduction in circulating lipid
concentration. No significant change in total cholesterol
or triglyceride levels was seen in concert with the
reduction in atherosclerosis, and the lipoprotein
profile of all 3 groups of mice was very comparable.
In contrast, knocking out the MyD88 gene and disrupting
immune signaling was associated with a significant
decrease in circulating levels of chemokines and pro-atherogenic
cytokines (MCP-1, IL-1, p40), which were significantly
reduced in the double-knockout mice, perhaps playing
a role in the atheroprotective effects of disrupting
innate immune signaling. TLR-4 and Apo E double-knockout
mice also demonstrate about a 25%-30% reduction in
atherosclerosis, parallel to that seen with the MyD88
knockout mice, as shown by Shah and colleagues.
|
PAGE
TOP
|
The adaptive
immune response |
|
In contrast to innate immunity, the adaptive immune
response (AIR) in atherosclerosis is more complicated.
AIR has the potential to be pro-atherogenic and anti-atherogenic.
AIR is generated when antigen-presenting cells,
such as dendritic cells, carry in antigen and expose
them to T-cells and B-cells, leading to activation
of the T and B cells. The T cells can then become
polarized, along the pro-inflammatory TH-1 phenotype
with release of interferon-gamma and pro-inflammatory
cytokines, or along the TH-2 phenotype with endo-inflammatory
cytokine IL-10 release and potential anti-atherogenic
effects. Similarly, B-cell engagement and activation
can lead to plasma cellformation and formation of
antibody to the presented antigen.
The auto-antigens that have elicited an AIR in experimental
animal studies and in humans include HSP-60, ß2GP1,
and ox-LDL, which is the most important and most studied.
The immune response to HSP-60 and HSP-65 is atherogenic,
as shown by immunization experiments in mice and rabbits
resulting in increased atherosclerosis. Conversely,
tolerization to HSP-65 using nasal or gastrointestinal
mucosal antigen exposure induces tolerance to HSP-65
and reduces atherosclerosis.
The immune response to ß2GP-1 is also atherogenic.
Immunization of experimental animals with ß2GP-1
is associated with accelerated atherosclerosis. Notably,
this effect can be transferred to non-immunized mice
through the transfer of lymphocytes from immunized
mice—indicating that this adaptive transfer conveys
the pro-atherogenic effects of the immune response
to ß2GP-1.
|
PAGE
TOP
|
Immune response
to oxidized LDL |
|
The evidence for the humoral immune response to ox-LDL
on atherosclerosis in humans had been conflicting.
Several studies suggested that in the absence of coronary
artery disease (CAD) there was less immune response
than in the presence of CAD. However, other studies
that examined different markers of atherosclerosis
showed no significant difference between controls
and cases for the magnitude of anti-oxidized LDL antibody
levels. Uncertainty about the immune response to oxidized
LDL wascreated.
The athero-protective effect of immunization with
oxidized LDL or native LDL was first shown by experiments
by Shah and colleagues and by Palinski and colleagues.
Confirmation came from other investigators in experiments
using LDL-R null mice and in another using apo-E null
mice.
Shah and colleagues studied the immune response
to ox-LDL in animal models. The rationale for the
experiments was that oxidative modification of LDL
leads to changes in the phospholipid and protein components
of LDL and thereby exposes neoantigens, which are
then recognized by the immune system and then can
react in the form of antibody production or T-cell
polarization and cytokine secretion.
Hypercholesterolemic rabbits were immunized with
LDL from rabbits as an antigen against homologous
unmodified LDL or homologous oxidative modified LDL.
After 16 weeks, in which the primary immunization
and a booster was given, no significant effect was
seen on circulating cholesterol levels. However, contrary
to expectations, both native and oxidized LDL as an
antigen dramatically reduced atherosclerosis, by nearly
60% in this rabbit model (p<0.05). About the same
time, nearly identical results were reported by Palinski
and colleagues, with about a 30%-40% reduction in
atherosclerosis in the aorta of the Watanabe rabbit
model, using malondialdehyde-modified LDL as an antigen.
|
PAGE
TOP
|
Immunotherapy
research in humans |
|
Apo B100-related peptide vaccines were developed
based on 101 peptide sequences that could have antibodies
in human serum, which were identified in a collaborative
program between Shah and colleagues and Nilsson and
colleagues in Sweden to develop a testable strategy
in humans.
In one experiment, vaccines were created using 2
peptide sequences with significant homology to mouse
Apo B100. Primary immunization then was given to Apo
E null mice at 6-7 weeks and a booster at 8-9 weeks.
At 25 weeks, the animals were sacrificed to examine
the extent of atherosclerosis. No statistically significant
difference was seen in serum cholesterol levels with
Peptide 1 or Peptide 2 compared to control, although
there was a trend for lower cholesterol levels in
the Peptide 1 group and a little higher in the Peptide
2 group compared to control (700 mg/dl, 1400 mg/dl,
and 1100 mg/dl, respectively). In contrast, about
a 50% reduction in atherosclerosis in the aorta was
found in the Peptide 2 group compared to the Peptide
1 group or control (p<0.01). Substantially less
atheroma production was found in the Peptide 2 group,
accompanied by a significant decrease in plaque inflammation
as measured by macrophage immunoreactivity, which
was reduced significantly in the Peptide 2 group.
In parallel, a significant increase in collagen staining
in the plaques was seen in the Peptide 2 group, as
measured by trichrome stain. Peptide 2 immunization
reduced atherosclerosis substantially, although the
cholesterol levels were the highest in this group,
reduced plaque inflammation, and increased plaque
collagen content, indicating a shift in the plaque
morphology to a more stable phenotype.
In terms of potential mechanisms, in splenic cytokine
expression, both peptide antigens caused an increase
in the TH-1 cytokine interferon-gamma, and TH-2 cytokines
IL-10 and IL-4. This suggests that the specific athero-protective
effects of Peptide 2 immunization are unlikely to
be mediated by a shift from a TH-1 to TH-2 cytokine
profile. In contrast, there was a significant difference
in the antibody levels produced in immunized groups.
Data from another set of experiments conducted with
2 additional athero-protective antigens (antigen 35
and antigen 74) showed that compared to control the
peptide-immunized animals had an increase IgG1 subtype
compared to IgG1 subtype, indicating a significant
humoral immune response to the antigen.
Intriguingly, adaptively transferred splenocytes
conveyed the atheroprotective effects of Peptide 2
immunization to non-immunized mice—highlighting
the important role of the spleen and splenocytes in
this process. In an experiment with 3 groups of non-immunized
mice, the mice that received splenocytes from Peptide
2 immunized mice had virtually the same degree of
reduction of atherosclerosis as the group that had
received the actual Peptide 2 immunization.
A passive immunization approach was used in recent
experiments, and confirmed that the effects of active
immunization could be reproduced by a passive immunization
using the pre-formed antibody against one of the protective
antigenic epitopes identified during the active immunization
experiments. In these experiments, compared to an
irrelevant antibody, the specific antibody in a dose-dependent
fashion reduced atherosclerosis in Apo E null mice,
while the irrelevant antibody had no significant effect.
Shah and colleagues have demonstrated the feasibility
and proof of concept that specific Apo B100-related
peptide sequences can promote athero-protective and
anti-inflammatory response in hypercholesterolemic
Apo E null mice. This response is associated with
increased antibodies, and can be adaptively transferred
through splenocytes. They believe that the potential
mechanism by which this immunization approach may
work is by creating antibodies that can lead to increased
clearance of LDL from the circulation and reduced
deposition of LDL within the atherosclerotic lesion.
Yet, the possibility that T-cell polarization with
TH-2 dominance may also be playing a role in reducing
atherosclerotic lesions cannot be ruled out.
|
PAGE
TOP
|
|
The immune system plays a complex role in atherosclerosis
with pro-atherogenic and athero-protective effects.
The innate immune system appears to be primarily pro-atherogenic,
while the adaptive immune response appears to have
both pro-atherogenic and athero-protective components.
Immunization using LDL or ox-LDL and specific Apo
B-100 related peptide sequences reduces atherosclerosis
and favorably modifies plaque composition, without
altering circulating levels of cholesterol, raising
the idea that immunotherapy is feasible. Immunotherapy
of atherosclerosis warrants further investigation.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2004
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|