|
|
ESC/JCS Joint Symposium |
| |
| Evidence-Based Medical Treatment of Hypertension |
|
| |
|
| Evidence-Based Medical Treatment of Hypertension |
| Adrian J. Brady, MD |
| Glasgow Royal Infirmary |
| Glasgow, UK |
| |
The European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) published their latest joint guideline for treating hypertension in 2007. Dr. Adrian J. Brady, Glasgow Royal Infirmary, Glasgow, UK, discussed the new recommendations in the guidelines.
The 2007 ESC/ESH guidelines state that the primary goal of hypertension treatment is maximum reduction in the long-term total risk of cardiovascular (CV) disease. Achieving this goal requires treating the raised blood pressure (BP) and all associated reversible risk factors. New areas of emphasis include prevention of atrial fibrillation, new onset diabetes, and unfavorable lipid effects. A key focus of the new guidelines is multiple drug therapy.
Most studies have shown benefits of treatment at BP <140/90 mmHg, with reduction in CV events continuing down to 110-115/70--75 mmHg. Consequently, the threshold for treatment of hypertension in the guidelines is sustained BP ≥140/90 mmHg with a target BP <140/90 mmHg. The treatment threshold and target are lower for high risk patients.
|
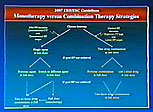
Figure 1. Strategies for monotherapy versus combination therapy.
【Click to enlarge】 |
|
|
The recommendations for treatment include lifestyle changes, including salt reduction, for all patients and institution of antihypertensive drug therapy. Thiazide diuretics, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers (ARBs), and beta blockers are all suitable agents. Because 50% of patients do not respond to the first drug, the emphasis has switched from sequential therapy to combination therapy. Use of more than one agent is necessary to achieve target BP in the majority of patients. A new recommendation is to initiate therapy with two drugs in patients with severe hypertension or marked CV risk (Figure 1). For patients with metabolic syndrome, an ACE inhibitor or ARB is recommended as first-line therapy.
|
Figure 2. Some usual combinations of some classes of antihypertensive drugs.
【Click to enlarge】 |
|
|
The VALUE and LIFE trials showed that ARBs provide better protection than other agents against atrial fibrillation (AF) in patients with hypertension, with the LIFE study showing a 33% reduction in AF with losartan. Studies also show that the new drugs (ACE inhibitors, ARBs, calcium channel blockers) cause less diabetes than the older drugs. Beta blockers, especially in combination with a diuretic, should not be used in patients with metabolic syndrome or at high risk of diabetes. Evidence based recommendations favor the use of ARBs, ACE inhibitors, and calcium channel blockers over the other drugs (Figure 2).
Dr. Brady concluded that the guidelines are moving in the direction of lower BP targets, reducing total CV risk, emphasizing combination therapy, and preventing disease progression. The most important factor is implementation—if the guidelines are implemented, strokes and heart attacks will be reduced. |
| |
| Page Top |
|
| |
|
| On-Going Japanese Clinical Trials for Hypertension: What Evidence We Need in Aging and Metabolic Era |
| Hiromi Rakugi |
| Osaka University Graduate School of Medicine, Osaka General Medical Center |
| Osaka, Japan |
| |
Several large randomized clinical trials designed to provide evidence for treatment of hypertension specific to the needs of the Japanese population were discussed by Dr. Hiromi Rakugi in this lecture.
Japan is experiencing a rapid increase in the elderly population and in obesity, both risk factors for hypertension. Metabolic syndrome also is an emerging risk factor in Japan. Hypertension affects more than 60% of elderly Japanese. Only half of these individuals are receiving therapy. While most hypertension-related deaths in the United States and Europe are due to ischemic heart disease, in Japan the main cause is stroke.
The Japanese Society of Hypertension (JSH) is supporting several large trials of hypertension therapy. In the CASE-J trial of candesartan versus amlodipine, the percentage of patients experiencing a first event (primary composite endpoints) by 48 months was the same in both treatment groups. Subanalysis showed that candesartan provided greater risk reduction in all-cause mortality and new-onset diabetes in obese patients.
The aim of the ongoing DIME trial is to confirm the safety of low-dose diuretics related to new-onset diabetes in 1,800 hypertensive patients. The JATOS trial of therapy with efonidipine in the elderly, compared a strict treatment group (target SBP <140 mmHg) and a mild treatment group (target SBP 14-160 mmHg). There was no difference in the cumulative incidence of the primary endpoint (cerebrovascular and cardiovascular mortality and morbidity and renal dysfunction). The VALISH trial of valsartan has a similar design and results so far show no difference between the strict and mild treatment groups.
|
Figure 1. The design of the Hypertension Objective Treatment based on Measurement by Electrical Devices of Blood Pressure Study being conducted in Japan.
【Click to enlarge】 |
|
|
|
Figure 2. Randomized controlled trials supported by the Japanese Society of Hypertension.
【Click to enlarge】 |
|
|
The PATE-Hypertension trial followed elderly hypertensive patients who responded to initial treatment with an ARB, ACE inhibitor, or calcium channel blocker. After 3-years follow-up, a J-shaped curve was observed, with increased cardiac events in patients who achieved SBP ≤120 mmHg.
The HOMED-BP study (N >4,000) aims to determine optimal target BP based on self-measured BP at home and optimal initial antihypertensive therapy (Figure 1). The HOSP trial (N=2,600) is evaluating the effects of amlodipine versus losartan, comparing modest (<140 mmHg) versus strict (<130 mmHg) home-measured target BP.
The COPE and COLM trials are evaluating optimal combination therapies to achieve strict BP control. The COPE trial is comparing the addition of thiazide diuretics, beta blockers, and ARBs to benidipine. COLM is comparing the addition of thiazide diuretics and calcium channel blockers to olmesartan.
The results of these trials (Figure 2) will provide useful information for revising Japan’s guidelines, concluded Rakugi. |
| |
| Page Top |
|
| |
|
| On-Going Japanese Clinical Trials for Hypertension II: Seeking for More Effective Pharmacological Treatment. The COmbination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) Trial. |
| Seiji Umemoto, MD, PhD |
| Yamaguchi University Hospital |
| Yamaguchi, Japan |
| |
Antihypertensive drug combinations are often necessary to achieve optimal blood pressure control but the effects of combination therapy have not been well studied with regard to morbidity and mortality. The objective of the ongoing COPE trial, presented by Dr. Seiji Umemoto, Yamaguchi University Hospital, is to determine which combination of drugs, when added to benidipine, is superior for achieving the targeted blood pressure (BP) and preventing cardiovascular events with the fewest adverse effects.
|
Figure 1. Design of the COPE Trial.
【Click to enlarge】 |
|
|
|
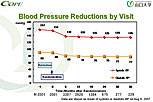
Figure 2. Blood pressure reductions by clinic visit in the COPE trial in Japan.
【Click to enlarge】 |
|
|
|
Figure 3: Achieved target blood pressure in the COPE trial.
【Click to enlarge】 |
|
|
Benidipine is a long-acting calcium channel blocker with renal protecting effects. Patients with BP ≥140/90 mmHg who required combination therapy were randomized to an angiotensin receptor blocker (ARB), beta blocker, or thiazide diuretic in addition to benidipine (Figure 1). The primary endpoints are a composite of fatal and non-fatal cardiovascular events and achievement of the target BP (<140/90 mmHg). The composite endpoint includes sudden death, fatal or nonfatal stroke, fatal or nonfatal myocardial infarction, hospitalization for unstable angina, new onset of heart failure, sudden cardiac death, new onset or worsening of peripheral artery disease, and new onset or worsening of renal failure.
As of November 30, 2006, 4,790 patients were enrolled in the trial. After a run-in phase, 3,501 were randomized to treatment. Of these, 3,433 patients received treatment and still were in the study as of July 2007. The patients were evaluated every 6 months for 3 years. The mean blood pressure of the overall study population dropped from 157/90 mmHg at baseline to 135/77 mmHg at 36 months (Figure 2). Six months after randomization, 55.5% of all patients achieved the target blood pressure. By 36 months, the percentage achieving the target blood pressure had increased to 65.9%, which is better than that achieved in usual care (Figure 3).
As of August 2007, trial investigators had reported a total of 75 cerebrovascular and cardiovascular events. These included:
- 27 cardiac events (8 non-fatal MIs, 13 hospitalizations due to angina, and 6 hospitalizations due to heart failure).
- 34 strokes (22 ischemic, 8 hemorrhagic, 4 transient ischemic attacks).
- 6 vascular events (5 peripheral artery disease, 1 aortic dissection).
- 1 case of new onset or worsening renal failure.
- 2 sudden deaths, and 10 other deaths.
The COPE trial will help clarify the questions of which antihypertensive combination confers the best protection against cardiovascular mortality and morbidity and which antihypertensive combination most effectively and safely achieves the target blood pressure.
|
| |
| Page Top |
|
| |
|
|