Dr. Masatsugu Hori, Chairperson of the 73th Annual Scientific Meeting of the Japanese Circulation Society, President of Osaka Medical Center, delivered the Presidential lecture on cardiovascular risk from the aspects of gene to population science.
Cardiovascular risk derived from genetic disorders and polymorphism
Analysis of 19 explanted hearts from patients who had heart transplantation revealed the marked deposit of triglyceride in the myocardium and coronary vessels of 2 (10%) patients. Genetic studies showed that both patients had homozygous for a point mutation in the adipose triglyceride lipase (ATGL) gene, which is essential for the hydrolysis of intracellular triglycerides. This abnormality was named as triglyceride deposit cardiomyovasculopathy.
In collaboration with the Institute of Physical and Chemical Research (RIKEN), Dr. Hori studied single nucleotide polymorphisms (SNPs) associated with myocardial infarction (MI). More than 65,000 SNPs associated with MI have been identified, with a functional SNP in lymphotoxin alpha (LTA) the most strongly associated. LTA is co-localized with the proteins galactin-2 and tubulin. Ozaki et al proposed that polymorphism of the galactin-2 gene (LGALS2) results in the overexpression of galactin-2 molecules, which bind with LTA and are transported on tubulin, resulting in enhanced production of LTA molecules. The LTAs activate the inflammatory response, increasing the risk for acute MI (AMI).
Multivariate analysis of atherosclerosis-related genes revealed that LTA polymorphism was significantly associated with increased risk of death (HR 2.46, 95% CI 1.24-4.86, p=0.010). An observational study found that MI patients (N=1807) with LTA polymorphism had a higher risk of death compared to those without the polymorphism (p=0.0410). However, this excess risk disappeared in patients treated with statins (N=583; p=0.088) probably due to their pleiotropic effects inhibiting the inflammatory response in the atheromatous plaque. Another study elucidated that patients with a combination of LTA, ABCA1, and ACE polymorphisms had as high as a 50% risk of MI whereas only a 20% risk in patients with LTA polymorphism alone (p<0.01).
Carviovascular risk derived from intracellular signal transactions
Activation of intracellular signaling pathways in response to neurohormonal activity, e.g. angiotensin II and sympathetic activation leads to contractile dysfunction, cell death, and ultimately to heart failure. Dr. Hori’s group recently identified a cellular pathway involving heparin-binding endothelial growth-like factor (HB-EGF). In this pathway, extracellular binding of activated ADAM12 with HB-EGF causes cleavage of the extracellular domain of HB-EGF, yielding soluble HB-EGF (sHB-EGF). sHB-EGF binds with the EGF receptor, resulting in cardiac hypertrophy and heart failure.
|
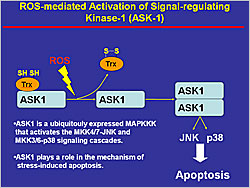
Figure 1. ROS-mediated Activation of Signal-Regulating Kinase-1 (ASK-1)
【Click to enlarge】 |
|
|
Among the signal transduction pathways that lead to cardiac hypertrophy, G-protein coupled receptor (GPCR) activation by angiotensin II induces reactive oxygen species (ROS) production in isolated cardiomyocytes. ROS activate apoptosis signal-regulating kinase-1 (ASK-1), which activates the MKK4/7-JNK and MKK3/6-p38 signaling cascades, resulting in increased apoptosis (Figure 1). In a study of ASK-1 knockout and wild-type mice, 4 weeks after MI the wild-type mice had cardiac remodeling whereas the knockout mice did not. In another study, administration of an dominant negative ASK-1, slowed the progression of disease, indicating that ASK-1 is a key molecule in the process of cellular injury in response to oxidative stress.
Myocardial ischemia followed by reperfusion in cyclophilin D (CypD) knockout and wild-type mice resulted in myocardial necrosis in the wild-type mice but there was no necrosis in the CypD knockout mice. Thus, Dr. Hori hypothesized that necrosis is not a passive cell death but may be regulated by a key molecule in the process of ischemic injury.
Stress on the endoplasmic reticulum (ER) can result in accumulation of unfolded proteins with ER dysfunction, leading to apoptotic cell death. The ER stress marker (GRP78) and the ER-initiated apoptotic signal (CHOP) are increased in myocardial tissue from patients with heart failure. Studies in mice showed that mechanical overload-induced heart failure is attenuated in CHOP knockout mice compared to wild-type mice. Accordingly, ER stress is indicated as an another cause of myocardial injury and heart failure.
Cardiovascular risk derived from population science
The Osaka Acute Coronary Insufficiency Study (OACIS) Group initiated an ongoing prospective study using an AMI registry in Osaka, Japan in 1998. A total of 9,098 cases were registered by March, 2009 and followed for up to 5 years; 92% of patients had at least a 1-year follow-up. A total of 5,200 serum samples and 4,200 DNA samples were collected.
|
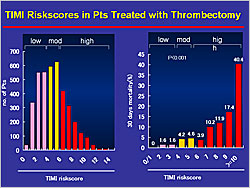
Figure 2. TIMI Risk Scores in Patients Treated with Thrombectomy
【Click to enlarge】 |
|
|
TIMI risk scores were used to determine prognosis with respect to 30-day mortality in the patients treated with thrombectomy. The prognosis was much worse for patients with high TIMI risk scores (6–≥10) compared to those with low (0–3) and moderate (4–5) scores (p<0.001) (Figure 2). No difference was observed in short-term mortality in patients in the low and moderate risk groups treated with or without thrombectomy. However, risk stratification by TIMI score showed that 30-day mortality rates were improved in patients in the high-risk group (9.2%) treated with thrombectomy versus high-risk patients not treated with thrombectomy (14.8%) (p=0.020). From these results, Dr. Hori concluded that thrombectomy can improve the prognosis of high risk patients.
A biomarker of cardic injury, serum cardiac troponin T (cTnT) typically increases in the acute phase of MI, returning to normal by about 10 days after MI onset. In the OACIS study, 39.1% of patients discharged without signs of myocardial ischemia still had slightly increased cTnT levels. Follow-up study showed that the patients with increased cTnT (>0.04 ng/mL, N=353) at discharge had a poor prognosis compared with patients with normal cTnT levels (<0.04 ng/mL, N=1,064) (p<0.01). However, no association between cTnT and NT pro-BNP indicates that the increased cTnT is not a sign of heart failure. Since endoscopic studies showed that 78.9% of patients with slightly increased cTnT still had thrombus in the coronary vessels, compared with only 26.2% of patients with negative cTnT (p<0.01). Thus, Dr. Hori hypothesized that the residual thrombus migrates to small peripheral coronary vessels, causing a microembolism, which is responsible for the cTnT release and the poor prognosis, since the observational study demonstrates that antiplatelet therapy in these patients improves their prognosis.
The Japan Atrial Fibrillation and Stroke Trial (JAST) evaluated the efficacy and safety of aspirin for the treatment of thromboembolism in patients with AF. The study enrolled 780 patients, with a follow-up of 3 years. The results showed that outcomes in the aspirin group were not improved over those in the control group that didn’t receive aspirin. The reason for this was increased bleeding events in the aspirin group (0.8%/year vs 0.2%/year in the control group). This is a good example of how population studies are important for guiding clinical practice.
Dr. Hori concluded that assessment of individual risk according to classic and genetic risk factors is extremely important, as is research into genetic/cellular mechanisms and signal transduction in cellular injury. Population studies are also important to verify risk at the population level and to evaluate therapeutic interventions.
|