|
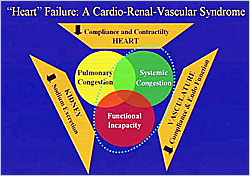
Figure 1. “Heart” Failure: A Cardio-Renal-Vascular Syndrome
【Click to enlarge】 |
|
|
Dr. Marvin A. Konstam (Tufts Medical Center, USA ) discussed the relationships between the heart, kidneys, and vasculature and the implications for the management of heart failure. Heart failure is a cardio-renal-vascular syndrome characterized by abnormalities of all three systems: abnormal cardiac compliance and contractility; abnormal sodium excretion in the kidneys; and abnormal structural and functional changes in the vasculature. Together these abnormalities produce the different heart failure syndromes, including pulmonary congestion, systemic congestion, and functional incapacity (Figure 1).
The SOLVD study showed that heart failure patients with the lowest glomerular filtration rates (GFR) had worse survival than patients with better renal function. Many studies on acute decompensated heart failure show that worsening renal function during hospitalization for heart failure correlates closely with adverse clinical outcomes. The ADHERE study demonstrated that abnormal BUN and creatinine are independent predictors of mortality during hospitalization. Worsening renal function during therapy for acute decompensated heart failure is associated with a poor prognosis.
Venous congestion, right heart failure, and increased right atrial and renal vein pressure have at least as much to do with progressive renal insufficiency in heart failure patients as renal perfusion. Ultrafiltration might be a better way to achieve normal fluid status in heart failure patients than loop diuretics, which can affect renal function. In the Unload study, heart failure patients were randomized to either ultrafiltration or diuretic therapy. Ultrafiltration removed more fluid and seemed to result in reduced hospitalizations for heart failure but it did not preferentially preserve renal function.
Some drug classes under investigation have the potential for preserving renal function while normalizing fluid status. One of these is selective A1 adenosine receptor antagonists. Adenosine reduces glomerular filtration pressure through its effects on the afferent artery and promotes renal tubular sodium reabsorption. A study of the adenosine receptor antagonist rolofylline showed that in heart failure patients with the same or greater degree of diuresis with a loop diuretic, there was a dose-related preservation of renal function. Patients who received the highest rolofylline doses had the greatest preservation of renal function. Ongoing studies are evaluating outcomes with adenosine receptor antagonist therapy.
Vasopressin antagonists are another potential therapeutic class. Most vasopressin antagonists under investigation, such as tolvaptan, are selective inhibitors of the V2 vasopressin receptor in renal tubules, which is responsible for free water reabsorption. A small randomized study showed worsening effective renal plasma flow (ERFP) and renal blood flow (RBF) with furosemide versus improved ERFP and RBF with tolvaptan.
Konstam et al performed a large set of studies (EVEREST trials) on tolvaptan in 4,000 patients. The results showed that at day 1, there was 0.7-0.9 kg greater fluid removal with tolvaptan compared to placebo (p<0.001), which was associated with a significant improvement in dyspnea (p<0.001). This benefit did not translate into improved long-term survival or hospitalization for heart failure, although these outcomes were not worsened. With chronic administration, normalization of fluid status was maintained with little or no differences in serum creatinine or BUN.
Patel et al studied endothelial function as determined by percent vasodilation in patients with ischemic and nonischemic dilated cardiomyopathy versus normal controls. In both the ischemic and nonischemic cardiomyopathy there was marked reduction in flow-mediated dilation, indicating endothelial dysfunction (p<0.01). Following cardiac transplantation, the percent vasodilation of nonischemic patients returned to normal but remained reduced in the ischemic patients, suggesting that the endothelial dysfunction in these patients was related to atherosclerosis rather than cardiomyopathy.
Many currently used therapies may improve outcomes by their vascular effects in addition to cardiac effects. The EPHESUS and RALES studies of aldosterone blockade showed a reduction in all cause mortality in heart failure patients. Two Japanese studies suggest that aldosterone blockade may improve the process of adverse ventricular remodeling in post-MI and chronic heart failure. A study by Konstam et al in chronic heart failure patients randomized to eplerenone or placebo did not demonstrate any significant remodeling in the eplerenone patients.
In another study, cardiac smooth muscle cells transfected with reporter for mineralocorticoid receptor (MR)-specific transcriptional effects showed a dose-related increase in MR-specific transcription with aldosterone administration. This effect indicates that MR receptors are present in the vasculature and influence gene expression. Genes upregulated by aldosterone include those for collagen types I and III and those responsible for deposition of vascular calcification and activation of immune mediated mechanisms within the vasculature. These results suggest that aldosterone has adverse effects in the vasculature.
Patients with reduced cardiac output and arterial pressure changes have increased turbulent flow along the endothelial margin and reversed flow in parts of the cardiac cycle, particularly in early diastole. An ex vivo study showed a linear increase in nitric oxide (NO) concentrations in the effluent as arterial flow increases. When the flow reverses, NO is not produced in the effluent. Retrograde flow causes increases in oxygen free radical production, which degrades the NO. This may be a hemodynamic link between heart failure and endothelial dysfunction.
In an endothelial remodeling experiment, laminar flow was introduced within an endothelial cell culture. Laminar flow was found to have effects on many genes, including genes that express endothelial cell cytoskeleton proteins. After 24 hours, micrographs showed a normal remodeling pattern in the endothelial cells under laminar flow, while cells in the static state were disorganized and lacked normal remodeling organization. These results suggest that changes in flow dynamics may be critically important in endothelial function and structure and potentially have anti-atherosclerotic and anti-thrombotic effects.
|
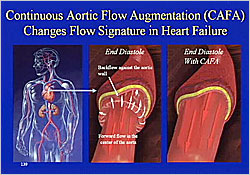
Figure 2. Continuous Aortic Flow Augmentation (CAFA) Changes Flow Signature in Heart Failure
【Click to enlarge】 |
|
|
A continuous aortic flow augmentation (CAFA) device superimposes laminar flow on top of pulsatile flow in the descending aorta, simulating the effects of exercise. In heart failure patients, CAFA changes the turbulent retrograde flow to normal forward flow (Figure 2). In the MOMENTUM study, 168 patients with acute decompensated heart failure were randomized to CAFA or control. In the control group, there was reduced pressure over 4 days due to diuresis but no increase in cardiac output. With the CAFA device there was reduced wedge pressure and increased cardiac index, mediated by a downstream vasodilation through mechanisms that are not clear. After 3 or 4 days with CAFA, patients had improved cardiac function.
Dr. Konstam concluded that abnormalities of the heart, vasculature, and kidneys conspire in producing chronic and acute heart failure. The high morbidity and mortality associated with heart failure exacerbation represents an important target for novel therapeutic interventions focusing on the heart, kidneys, and blood vessels.
|