|
|
Symposium 1 |
| |
| Biomarkers, Genetic Polymorphisms, and Risk Estimation |
|
| |
|
| Biomarkers and Cardiovascular Disease Prevention: Key Steps Toward Personalized Medicine. |
| Daniel Levy |
| Framingham Heart Study, Boston University School of Medicine, Harvard Medical School, Boston, Massachusetts, USA |
| |
Dr. Daniel Levy, Framingham Heart Study, presented this Keynote Lecture on the use of biomarkers to prevent, diagnose, and treat cardiovascular disease (CVD). A biomarker is a characteristic that can be objectively measured and evaluated as an indicator of genotype, normal biological processes, pathological processes, or response to therapeutic intervention.
Traditional approaches for biomarkers are based on understanding the risk factor contributions to the biology of cardiovascular disease (CVD), using information from basic science, population science, and large-scale clinical trials of interventions. For example, cholesterol has been known to be associated with atherosclerosis since 1859. Studies in the 1980s showed that lowering cholesterol reduces risk of CVD, leading to the widespread use of statin therapy.
Future strategies will be technology driven and include genetics and genomics; proteomics, metabolomics, and lipomics; and bioinformatics, systems biology, and data mining. These strategies will be integrated to rapidly identify causal CVD risk factors. Already, technological advancements allow genome-wide screening that can test 1 million genetic variants in a single DNA specimen. For example, multiple genome wide studies have identified a region on chromosome 9 associated with myocardial infarction (MI). Future studies will provide information on the contribution of this region to MI.
The Framingham SNP-Health Association Resource (SHARe) project has genotyped 550,000 SNPs in 9,000 Framingham Heart Study participants from three generations. From this a database comprising 5 billion genotypes and associated clinical patient data has been created for research purposes.
|
Figure 1. Advances in technology towards proteome.
【Click to enlarge】 |
|
|
|
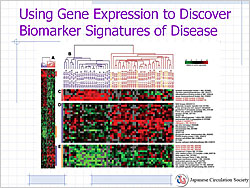
Figure 2. Using gene expression to discover biomarker signatures of disease.
【Click to enlarge】 |
|
|
As research moves from the genome to the transcriptome to the proteome, the medical community moves closer to understanding the biology of disease (Figure 1). Future studies will use high-throughput technology to measure large numbers of biomarkers, with discovery proteomics, metabolomics/lipomics, genome-wide association, and gene expression profiling studies. Gene expression profiling is now available to study the regulation of all 30,000 genes in the human genome to further understanding of the biomarker signatures of disease (Figure 2).
When data from genetic variation, gene expression, and circulating biomarkers are combined and related to clinical disease in populations, the biology of disease and causal pathways will be better understood. Combining genomics, biomarker studies, and systems biology will provide additional insights into the pathogenesis of CVD. Development of new diagnostic tests and identification of new therapeutic targets will lead to the realization of personalized medicine, the use of information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease.
|
| |
| Page Top |
|
| |
|
| ST2 is an Emerging Functional Biomarker Predicting Mortality after Acute Heart Events and Comprising Stress-Induced Cardioprotective Signaling System with IL-33. |
| Shoji Sanada |
| Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA; National Cardiovascular Center, Suita, Japan |
| |
|
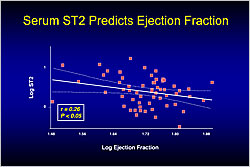
Figure 1. Serum ST2 Predicts Ejection Fraction.
【Click to enlarge】 |
|
|
Dr. Shoji Sanada, National Cardiovascular Center, Suita, Japan, discussed the roles of ST2 and IL-33 after acute cardiac events. ST2 is an IL-1 receptor (IL-1R) expressed in the heart. Previous studies reported that ST2 is markedly induced in mechanically overloaded cardiomyocytes. In a rat model of MI, higher serum ST2 levels 24 hours after myocardial infarction (MI) significantly predicted lower ejection fraction after 30 days (p<0.05) (Figure 1).
In a study of 810 patients with acute MI, patients with higher serum ST2 levels at presentation had a significantly higher mortality rate 30 days after MI onset (p=0.0009). In another study, ST2 levels of 448 patients with acute heart failure (HF) were measured and grouped into quartiles. After 30 days, there was a significant increase in all-cause death rates (p=0.01) and HF death rates (p=0.008) in the higher quartiles. Another study found that both ST2 and NT-proBNP levels 24 hours after admission for acute HF had similar value in predicting cardiovascular death and HR recurrence.
|
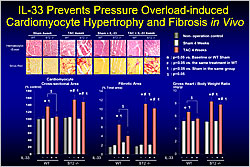
Figure 2. IL-33 Prevents Pressure Overload-Induced Cardiomyocyte Hypertrophy and Fibrosis In Vivo.
【Click to enlarge】 |
|
|
In 2005, IL-33 was identified as a functional ligand for ST2. In 2007, Dr. Sanada found that IL-33 protein production was induced by mechanical stress predominantly in cardiac fibroblasts but also in cardiomyocytes. IL-33 was found to transiently activate NF-kB in cardiomyocytes and cardiac fibroblasts but it attenuated NF-kB activation by AT-II and other GPCR agonists. Transverse aortic constriction (TAC) for 4 weeks in ST2 knockout mice resulted in increased cardiomyocyte cross-sectional area and increased fibrosis versus wild-type (WT) mice. Daily administration of IL-33 protein reduced both changes in WT mice but not in ST2 knockout mice (Figure 2).
IL-33 blocked TAC-induced NF-kB activation only in WT mice. TAC induced more cardiac remodeling in ST2 knockout mice versus WT mice. IL-33 treatment restored all parameters and improved survival in WT mice but not in ST2 knockout mice (p<0.05), suggesting that IL-33 functions through the ST2 receptor. IL-33-treated rats had significantly reduced infarct size and attenuated fibrosis after MI versus controls. IL-33 also eliminated apoptosis in the border zone after MI (p<0.01). IL-33 treatment reduced cardiac remodeling in an MI mouse model after 4 weeks but not in ST2 knockout mice. Kaplan-Meier analysis showed that IL-33 improved survival after MI in WT mice but not in ST2 knockout mice (p<0.05).
Dr. Sanada concluded that ST2 is a promising biomarker for predicting mortality after acute cardiac events. These studies showed that IL-33 is cardioprotective through the ST2 receptor.
|
| |
| Page Top |
|
| |
|
| Transcardiac Increase in Norepinephrine and Prognosis in Patients with Chronic Heart Failure–Comparison with Cardiac I-123-metaiodobenzylguanidine Imaging. |
| Takayoshi Tsutamoto |
| Department of Cardiovascular Medicine, Shiga University of Medical Science, Japan |
| |
Dr. Takayoshi Tsutamoto, Shiga University of Medical Science, presented this study, which evaluated the prognostic role of the transcardiac gradient of norepinephrine (NE) in patients with chronic heart failure (CHF). The hemodynamic parameters and plasma levels of NE, brain natriuretic peptide (BNP), and N-terminal proBNP (NT-proBNP) were measured in the aortic root (AO) and coronary sinus (CS) of 356 patients with CHF. The patients were followed for a median 3.5 years.
|
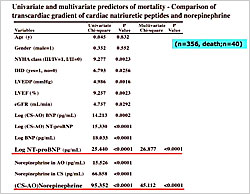
Figure 1. Univariate and multivariate predictors of mortality – Comparison of transcardiac gradient of cardiac natriuretic peptides and norepinephrine.
【Click to enlarge】 |
|
|
There were 40 cardiac deaths during the follow-up period. After adjustment for clinical variables associated with CHF, the transcardiac gradient of NE (p<0.0001) and plasma log NT-proBNP levels (p<0.0001) were independent predictors of mortality (Figure 1). A high log NT-proBNP was an independent predictor of cardiac death even after considering the transcardiac increase in BNP and NT-proBNP. A transcardiac increase in NE was an important predictor of cardiac mortality, suggesting the importance of cardiac sympathetic activity in CHF.
The patients were divided into four groups based on the cutoff levels of NT-proBNP and transcardiac gradient of NE: Group 1– NT-proBNP<796 pg/mL, ∆NE<108 pg/mL (N=144); Group 2– NT-proBNP<796 pg/mL, ∆NE>108 pg/mL (N=75); Group 3– NT-proBNP>796 pg/mL, ∆NE<108 pg/mL (N=68); Group 4– NT-proBNP>796 pg/mL, ∆NE>108 pg/mL (N=69). Patients in Group 1 had a hazard ratio (HR) for death of 5.32 versus those in Group 2 and an HR of 14.32 versus patients in Group 4 (p<0.0001).
|
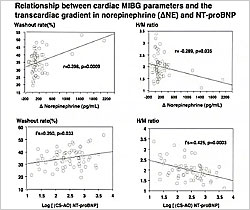
Figure 2. Relationship between cardiac MIBG parameters and the transcardiac gradient in norepinephrine (∆NE) and NT-proBNP.
【Click to enlarge】 |
|
|
Iodine-123-meta-iodobenzylguanidine (MIBG) imaging was used to assess sympathetic activity in 67 of the patients. The MIBG results were compared with the transcardiac gradient of NE, BNP, and NT-proBNP. The transcardiac increase in NE significantly correlated with the MIBG washout rate (p=0.009) and heart/mediastinum (H/M) ratio (p=0.035) (Figure 2). Of these 67 patients, 17 died. On univariate analysis, the MIBG parameters washout rate (p=0.0093) and H/M ratio (p=0.0224) were predictors of mortality in these patients. On multivariate analysis, the transcardiac increase in NE (p=0.0025) and the plasma log NT-proBNP levels (p=0.0282) were independent predictors of mortality but MIBG parameters were not.
In summary, after adjusting for clinical variables associated with CHF, the transcardiac gradient of NE and plasma log NT-proBNP were independent prognostic predictors in patients with CHF. Among patients in whom MIBG was performed, the transcardiac increase in NE was a better predictor of mortality than MIBG parameters. Dr. Tsutamoto concluded that the transcardiac gradient of NE is an independent and useful predictor for evaluating the prognosis of patients with CHF.
|
| |
| Page Top |
|
| |
|
|