|
|
Symposium 2 |
| |
| Current Status and Future Perspective of Antiplatelet Therapy in Management of Coronary Artery Disease |
| Current Status and Future Perspective of Antiplatelet Therapy in Management of Coronary Artery Disease. |
| David J. Moliterno, MD |
| Division of Cardiovascular Medicine, University of Kentucky, Kentucky, USA |
| Optical Coherence Tomography Study of Neointimal Coverage after Sirolimus Eluting Stent Implantation–Implications for the Long-Term Dual Antiplatelet Therapy. |
| Shiro Uemura |
| Nara Medical University, Kashihara, Japan |
| Clopidogrel Resistance in Japanese Patients. |
| Hisanori Horiuchi |
| Department of Cardiovascular Medicine, Kyoto University, Japan |
| Development of Computer Based Virtual System to Predict Antithrombotic Effects of Newly Developed Antiplatelet Agents. |
| Shinya Goto |
| The Institute of Medicine, Tokai University, Japan |
|
| |
|
| Current Status and Future Perspective of Antiplatelet Therapy in Management of Coronary Artery Disease. |
| John H. Alexander, MD, MHSc, FACC |
| Duke University Medical Center, Durham, North Carolina, USA |
| |
Dr. John H. Alexander discussed the current issues and future strategies of antiplatelet therapy for coronary artery disease. Boersma’s review of GP IIb/IIIa inhibitor trials in patients with non-ST elevation acute coronary syndromes found consistent reductions in death and non-fatal MI, except for the GUSTO-IV trial of abciximab, which showed no benefit. A review of oral GP IIb/IIIa inhibitor trials found that these agents were associated with worse outcomes, leading to the conclusion that low and variable GP IIb/IIIa inhibition is harmful.
Clopidogrel is a highly effective oral ADP antagonist but a recent study reported risk of rebound after discontinuation. The risk for recurrent ischemic events is higher early on after discontinuation than in the long-term.
|
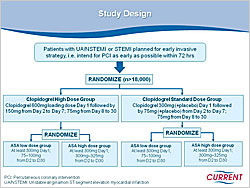
Figure 1. Design of the CURRENT Study.
【Click to enlarge】 |
|
|
Optimal dose and duration are continuing issues in antiplatelet therapy. An overview of aspirin trials found benefit at 75-1,500 mg/day doses. Trials of clopidogrel dosing reported that higher doses produce faster and greater inhibition of platelet aggregation. The CURRENT study is investigating higher doses of clopidogrel for short-term therapy and subsequent low-dose versus high-dose aspirin in over 18,000 patients (Figure 1). The CHARISMA trial of long-term clopidogrel use in patients with prior MI found that within 30 months follow-up clopidogrel continued to have benefit (p=0.031).
Serebruany reported inter- and intra-patient variability in response to a fixed dose of clopidogrel. Factors affecting level of platelet inhibition include compliance, absorption, metabolism, secretion, receptor number and function, and baseline platelet activity levels. Ongoing studies are evaluating the role of platelet function testing for individualizing antiplatelet therapy. A study of genotype and clopidogrel interactions found that patients with allelic variants of ABCB1 and CYP2C19 had lower exposure to the active metabolite of clopidogrel and higher rates of ischemic events than patients without these variants.
Data from the GRACE Registry suggest that MI patients have major bleeding rates (4.5%). Predictors of major bleeding are the same as those for ischemic events. Thus, patients with the most to gain from antiplatelet therapy are also at the highest risk of bleeding. Patients with major bleeding have higher mortality (15.3%) than those without major bleeding (5.3%). The TRITON study of clopidogrel versus prasugrel showed that prasugrel is more effective but causes more bleeding.
|
Figure 2. Phase 3 Clinical Trials of Novel Antiplatelet Regimens Compared to Clopidogrel.
【Click to enlarge】 |
|
|
Current antiplatelet therapy is suboptimal, with problems including recurrent ischemic events and bleeding complications. New antiplatelet agents and smarter use of existing therapies have the potential to improve both ischemic and bleeding outcomes. Several ongoing trials are comparing novel antiplatelet regimens with clopidogrel (Figure 2).
|
| |
| Page Top |
|
| |
|
| Current Status and Future Perspective of Antiplatelet Therapy in Management of Coronary Artery Disease. |
| David J. Moliterno, MD |
| Division of Cardiovascular Medicine, University of Kentucky, Kentucky, USA |
| |
Dr. David J. Moliterno, MD, University of Kentucky, presented an overview of antiplatelet therapy trial data in patients with acute coronary syndromes (ACS). The ACC/AHA 2007 guidelines for ST-elevation myocardial infarction (STEMI) recommended: adding clopidogrel 75 mg/day to aspirin in STEMI patients; 300 mg clopidogrel loading dose in patients <75 years; long-term therapy (1 year) with clopidogrel; and starting abciximab as early as possible before primary PCI.
New data have emerged since the guidelines were updated. In the FINESSE trial of patients who received either abciximab alone or in combination with fibrinolytic therapy and aspirin or heparin had similar rates of all-cause mortality and death, CHF, VF, or shock. However, bleeding was higher in the combination group (p<0.001).
In the HORIZONS trial, a clopidogrel 600 mg loading dose resulted in significantly reduced bleeding and cardiovascular events versus a 300 mg loading dose. In the BRAVE-3 trial, STEMI patients receiving abciximab after a 600 mg clopidogrel loading dose plus aspirin or heparin had no benefit in 30-day mortality versus patients receiving placebo instead of abciximab.
For non-STEMI patients, the 2007 guidelines emphasize a need for antiplatelet therapy with clopidogrel or a GP IIb/IIIa inhibitor in addition to aspirin. Several new agents are emerging for non-STEMI patients. The TRITON-TIMI 38 trial in ACS patients showed that prasugrel reduced MI rates (p<0.001) but increased bleeding (p=0.002) versus clopidogrel. Prasugrel reduced stent thrombosis up to 30 days (p<0.0001) and from 30-450 days (p=0.03) versus clopidogrel. The CHAMPION trial is evaluating the IV reversible P2Y12 inhibitor cangrelor versus clopidogrel in ACS patients. The PLATO trial is studying the oral reversible agent AZD6140 versus clopidogrel.
|
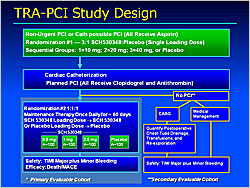
Figure 1. Design of the TRA-PCI Study.
【Click to enlarge】 |
|
|
|
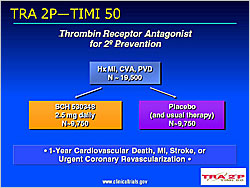
Figure 2. Design of theTRA 2P–TIMI 50 Study.
【Click to enlarge】 |
|
|
The TRA-PCI study (Moliterno et al) evaluated the oral, potent, selective thrombin receptor antagonist (TRA) SCH530348 versus placebo (Figure 1). TRA was not associated with increased TIMI or non-TIMI bleeding. A 40 mg loading dose achieved ≥80% TRAP-induced platelet aggregation (IPA) in 1-2 hours in 68-96% of subjects. Maintenance doses of 1 mg and 2.5 mg sustained ≥80% IPA at 30 and 60 days in all subjects. SCH530348 was associated with decreased death/MACE and MI (p=NS). SCH530348 is being evaluated in the Japanese TRA-ACS trial, the global TRACER trial, and the TRA 2P-TIMI 50 trial (Figure 2).
Dr. Moliterno concluded that antiplatelet therapy remains crucial in patients with ACS. Antithrombotic therapy options are steadily increasing. PAR-I (platelet-thrombin) drugs are progressing in ACS and secondary prevention trials.
|
| |
| Page Top |
|
| |
|
| Optical Coherence Tomography Study of Neointimal Coverage after Sirolimus Eluting Stent Implantation–Implications for the Long-Term Dual Antiplatelet Therapy. |
| Shiro Uemura |
| Nara Medical University, Kashihara, Japan |
| |
Restenosis and repeat target lesion revascularization rates have been greatly reduced with drug eluting stents (DES) compared to bare metal stents (BMS). Recently, reports of increased incidence of late stent thrombosis with DES have emerged. Late stent thrombosis usually occurs more than one year after implantation and is associated with discontinuation of antiplatelet therapy. Therefore, the current ACC/AHA/SCAI Guidelines recommend longer term antiplatelet therapy with aspirin and clopidogrel after DES implantation.
Because neointima formation over the DES struts is much thinner than on BMS, it cannot be visualized with conventional angiography or intravascular ultrasound (IVUS). However, the neointima on the DES struts can be clearly seen with optical coherence tomography (OCT). The purpose of this study, presented by Dr. Uemura, Nara Medical University, was to analyze the time course of neointimal growth over sirolimus-eluting stent (SES) struts and identify factors influencing the delayed neointimal growth on SES.
Patients (N=60) with previously implanted SES were grouped according to follow-up time: G1 <8 months (N=27, 28 SES); G2 9-24 months (N=18, 21 SES); G3 >25 months (N=15, 17 SES). The mean follow-up was 6.6 months in G1, 13.8 months in G2, and 32.6 months in G3. Baseline characteristics were similar among the groups. Cross-sectional OCT images were analyzed at 1 mm intervals from the distal to the proximal end of the stent.
|
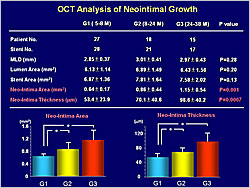
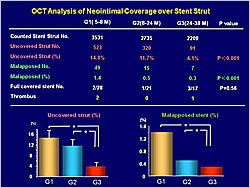
Figure 1. The optical coherence tomography analysis of neointimal growth.
【Click to enlarge】 |
|
|
|
Figure 2. The optical coherence tomographys analysis of neointimal coverage over the stent strut.
【Click to enlarge】 |
|
|
OCT analysis showed significant increases in neointimal area (p=0.001) and thickness (p=0.0007) with longer follow-up (Figure 1). The number of uncovered (p<0.001) and malapposed (p<0.001) struts decreased with longer follow-up (Figure 2).
Patients with diabetes had a higher percentage of uncovered stent struts than those without diabetes (19.6% vs. 13.2%, p=0.03). Dyslipidemia, hypertension, and smoking did not influence strut coverage. Small diameter stents were associated with a higher rate of uncovered struts than larger diameter stents (19.5% vs. 11.3%, p=0.003). Stent struts located on either calcified or lipid plaques had a higher rate of uncovered struts than those on fibrous plaques (p=0.002).
Dr. Uemura recommended cautious use of DES because the precise mechanisms of late thrombosis are unknown. Longer duration dual antiplatelet therapy appears to be safer than short-term therapy. A BMS should be used in patients with poor drug compliance, bleeding risk, near future surgery planned, and in emergency cases. The next generation of DES, such as those with biodegradable polymers, non-polymeric drug elution, or absorbable stent platforms may resolve the complication of late stent thrombosis.
|
| |
| Page Top |
|
| |
|
| Clopidogrel Resistance in Japanese Patients. |
| Hisanori Horiuchi |
| Department of Cardiovascular Medicine, Kyoto University, Japan |
| |
Coronary stent thrombosis is a serious complication that can be prevented with dual antiplatelet therapy with aspirin and an ADP receptor blocker. The ADP receptor blockers available in Japan are ticlopidine and clopidogrel. Ticlopidine is dosed at 500 mg/day in the U.S. but only at 200 mg/day in Japan. The standard clopidogrel dose currently is 75 mg/day in both countries, leading some Japanese clinicians to wonder if this dose is too strong for the Japanese. Dr. Hisanori Horiuchi, Kyoto University, explored this issue by evaluating the efficacy of clopidogrel in 30 Japanese patients scheduled for PCI.
The patients received a 300 mg loading dose of clopidogrel on the first day followed by 75 mg/day with low-dose aspirin. Platelet aggregation was measured using ADP as a stimulus.
Platelet aggregation decreased with clopidogrel intake in a time-dependent manner, reaching a plateau at 48 hours. When compared with results from a similar protocol in American patients, the clopidogrel effect in Japanese patients was weaker. Among the Japanese patients, 14% were nonresponders, 50% were “hypo-responders,” and 36% were good responders.
|
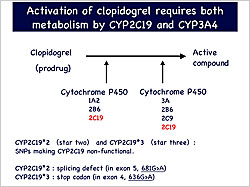
Figure 1. Activation of clopidogrel requires metabolism by both CYP2C19 and CYP3A4.
【Click to enlarge】 |
|
|
Clopidogrel is a prodrug that undergoes two-step metabolism by CYP2C19 and CYP3A4 to produce its active compound. Two frequent mutations in Asian populations, star 2 and star 3, inactivate CYP2C19 (Figure 1). Genotyping CYP2C19 in 25 patients showed that 24% had homozygous mutations, resulting in inactive CYP2C19, while 32% were heterozygous and 44% were wild-type. Clopidogrel was less effective in patients with homozygous (p<0.05) and heterozygous (p<0.05) mutations than in those with the WT enzyme. In contrast, 80% of Americans have wild-type CYP2C19 and experience 30-40% effectiveness with clopidogrel, similar to that of Japanese with the wild-type enzyme.
Three recent studies showed that CYP2C19 mutations are associated with cardiovascular events after coronary stenting. In one study, 2.6% of CYP2C19 mutants had stent thrombosis versus 0.8% of wild-type patients (p=0.02).
|
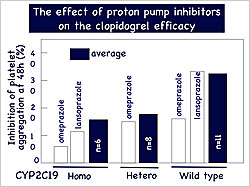
Figure 2. The effect of proton pump inhibitors on clopidogrel efficacy.
【Click to enlarge】 |
|
|
Proton pump inhibitors (PPIs) are recommended for patients receiving dual antiplatelet therapy with aspirin and clopidogrel. Some PPIs are inactivated by CYP2C19, so concomitant administration with clopidogrel might competitively inhibit clopidogrel. In patients with CYP2C19 mutations, the effect of this drug interaction would be stronger. Among the 25 patients in Dr. Horiuchi’s study, 5 were on PPIs. These patients had similar or lower effectiveness with clopidogrel compared to those not taking PPIs (Figure 2).
Dr. Horiuchi concluded that the lower response to clopidogrel in Japanese patients might be due to CYP2C19 mutations and interactions with CYP2C19-metabolized drugs. |
| |
| Page Top |
|
| |
|
| Development of Computer Based Virtual System to Predict Antithrombotic Effects of Newly Developed Antiplatelet Agents. |
| Shinya Goto |
| The Institute of Medicine, Tokai University, Japan |
| |
Dr. Shinya Goto, Tokai University, developed a virtual model of platelet function, focusing on the two major platelet-specific membrane proteins–glycoprotein (GP) 1ba and GP IIb/IIIa. The virtual platelets adhere on platelets and von Willebrand factor (VWF) through virtual springs with different binding constants. The binding constant of each spring on each platelet was designed to: increase under shear stress conditions, mimic GP1ba function; increase upon platelet activation, mimic GP IIb/IIIa function; and be influenced by the platelet’s virtual location. The process of platelet activation was modeled, from Grade 0–quiescent platelets–through Grade 5–irreversible adhesion with fibrin formation. The behavior of the virtual platelets in a virtual vessel was calculated using the Navier-Stokes Equation.
The accuracy of the model was validated using a parallel plate flow chamber system with human platelets. In real human platelets, stable adhesion on VWF was inhibited with P2Y12 receptor blockade. The virtual system can predict this behavior without using human blood.
The virtual system was used to predict platelet thrombus formation on a stent. Virtual stents with different shaped struts were modeled implanted in blood vessels with virtual blood flow. The model showed that thrombogenicity around stents is increased by blood flow disturbance caused by the stent struts. Virtual platelet accumulation on injured vessels was enhanced by the presence of a stent strut, with the platelets adhering downstream of the strut.
The stent diameter was an important determinant of platelet accumulation—the intermediate size (1mm) was more thrombogenic than the narrow ( 0.5 mm) and wide (2 mm) stents. Thrombogenicity also was greater with constant blood flow compared with pulsatile flow.
|
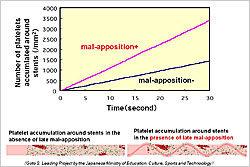
Figure 1. Platelet accumulation in presence and absence of malapposition.
【Click to enlarge】 |
|
|
Late malapposition is thought to be a contributing factor in increased thrombogenicity in patients with drug-eluting stents (DES). When malapposition was modeled, the virtual system showed much greater platelet accumulation around the stent in the presence of malapposition than in its absence (Figure 1).
Dr. Goto expanded the model from a simple particle with adhering function to a cell with activation signals and metabolic processes. The model includes initiation of the activation signal with VWF, mitochondria providing intracellular energy, and localization of activated GP IIb/IIIa. Simulation of moving platelets showed the process of activation, adherence, and thrombus formation.
Dr. Goto plans to develop a complete virtual system to screen new antiplatelet agents, maximize safety and efficacy of available agents, and to predict the safety and efficacy of specific agents in specific individuals.
|
| |
| Page Top |
|
| |
|
|