 |
|
|
 |
| Prediction and Prevention of Restenosis
After Angioplasty |
|
Teruo Inoue, M.D.
Koshigaya Hospital,
Dokkyo University
Atsushi Hirayama, M.D.
Cardiovascular Division,
Osaka Police Hospital
Junko Honye, M.D.
Second Department
of Internal Medicine, Nihon University
Richard E. Kuntz, M.D.
Brigham & Women's
Hospital, Boston, Massachusetts. |
|
|
|
 |
|
|
 |
|
Restenosis after coronary
angioplasty is the major limitation of percutaneous
coronary intervention (PCI). Stent deployment reduces
restenosis, therefore, stents are now used in up to
70% of PCI cases in Japan. Recurrence of restenosis
after stent implantation—in-stent restenosis—is
still observed, however, in 20-30% of procedures. Restenosis,
therefore, remains the “Achilles heel” of
PCI and represents a vexing new problem with no easy
solution.
At this symposium, specialists in the field discussed
the prediction and prevention of restenosis, focusing
on new predictive markers and strategies for managing
this troublesome occurrence. |
|
Causes of Restenosis and Predictive Biomarkers |
Restenosis is a unique vascular
expression of the local wound-healing response after
balloon-induced injury by angioplasty. This response
to injury is characterized by a sequence of inflammation,
granulation, extracellular matrix remodeling, and smooth
muscle cell proliferation. These processes lead to neointimal
hyperplasia—the most important mechanism underlying
restenosis after stents are implanted.
The local inflammatory response to percutaneous coronary
angioplasty (PTCA) produces an elevation in C-reactive
protein and interluekin-6, which can serve as possible
predictors of restenosis. In addition to the release
of these inflammatory markers, the process of neointimal
hyperplasia also involves the activation of platelets,
leukocytes and vascular endothelial cells. Adhesion
molecules mediate the interaction of these factors and
their expression can be demonstrated after angioplasty
and also in association with restenosis. In fact, certain
adhesion molecules may prove to be predictive of restenosis,
according to Teruo Inoue, of Koshigaya Hospital, Dokkyo
University. |
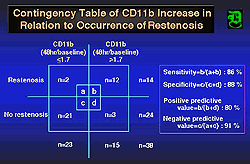 |
| Figure
1. The percent change from baseline in the molecule
CD11b was predictive of the late loss index after
angioplasty. |
| Click
to enlarge |
|
Using flow cytometric
analyses before and after PTCA, Inoue and colleagues
have studied the expression of Mac-1 (CD11b/CD18) and
L-selectin (CD62L) on the surface of neutrophils, and
the platelet membrane surface glycoproteins P-selectin
(CD62P) and CD63. Significant changes in these adhesion
molecules were demonstrated 24 and 48 hours after the
procedure, most prominently in patients who received
stents (compared to conventional PTCA) and in patients
who went on to develop restenosis, he reported. For
two molecules—CD11b and CD18—the percent change
from baseline predicted late loss index after angioplasty
(Figure 1). “Mac-1 upregulation can be observed
to predict restenosis, even in the peripheral blood
sample,” Dr. Inoue noted. |
In both balloon angioplasty
and coronary stenting, the most powerful predictor of
restenosis was neutrophil CD11b 48 hours after the procedure.
The sensitivity of this marker was 86%, the specificity
was 88%, the positive predictive value was 80%, and
the negative predictive value was 91%. “These numbers
are very, very high,” he remarked.
The key point, according to Dr. Inoue, is that the kinetics
of platelet and leukocyte activation mediated by cell
adhesion molecules after coronary angioplasty can predict
subsequent restenosis. In the future, pharmacologic
approaches targeting adhesion molecules [possibly, glycoprotein
2b/3a inhibitors like abciximab] may be a “powerful
strategy” for preventing restenosis. In this setting,
flow cytometric analysis of adhesion markers could be
used to verify therapeutic efficacy. |
PAGE
TOP
|
New Technologies
to Predict Restenosis |
| Factors pertaining to plaque color grade, coronary
anatomy, and coronary circulation may also predict the
risk of restenosis after PTCA, according to information
provided by new technologies. One new technology, coronary
angioscopy, is a powerful tool to characterize plaque
and thrombus and possibly relate such characteristics
to restenosis risk, according to Atsushi Hirayama, of
the Cardiovascular Division of Osaka Police Hospital. |
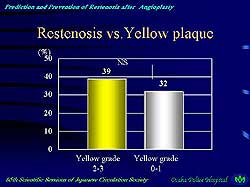 |
| Figure
2. Yellow plaques were found to be more frequent
in restenotic lesions compared to original lesions.
|
| Click
to enlarge |
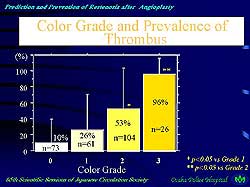 |
| Figure
3. The magnitude of thrombus accelerates further
thrombus formation and enhances plaque proliferation.
|
| Click
to enlarge |
|
Hirayama described
a study of 111 de novo lesions observed by angioscopy
before intervention, 38 of which developed restenosis.
The study evaluated the plaque color and amount of thrombus
in the restenotic lesions and found yellow plaques to
be more frequent and the amount of thrombus to be greater
in the restenotic, versus original, lesions (Figure
2). He also described studies suggesting that the magnitude
of thrombus in these target lesions accelerates further
thrombus formation and enhances plaque proliferation
(Figure 3).
Several other technologies are yielding very useful
predictive information. Intravascular ultrasound (IVUS),
coronary flow velocity, and pressure measurements, in
particular, have demonstrated predictive value in several
studies.
Junko Honye, MD, of Nihon University, said IVUS-guided
angioplasty has been shown to result in a target vessel
revascularization rate of less than 9% at 9 months.
And lumen cross-sectional area and amount of residual
plaque after stent implantation, which can be determined
by IVUS, have proven to be strong predictors of restenosis.
In a study of 2,343 stented lesions, for example, the
IVUS stent-to-lumen cross-sectional area was the best
predictor of in-stent restenosis. The lumen cross-sectional
area after stenting should be 7 or higher, in order
to reduce the restenosis rate, studies suggest.
Coronary flow reserve (CFR) using flow wire is also
predictive of restenosis. In the European DEBATE trial,
target lesion revascularization rate dropped to 16%
in procedures achieving CFR > 2.5 and angiographic
percent diameter stenosis < 35%. The pressure-guided
fractional flow reserve (FFR) measurement can also be
predictive, she added, noting, “The better the
FFR post-procedure, the less the restenosis rate. The
endpoint should be > 0.95.” |
In addition, Dr. Honye also advised cardiologists
to select the right size balloon and pressure. “When
the pressure is too high or when you use a larger size
balloon aggressively, sometimes that promotes neointimal
hyperplasia. You should simply not try to open up the
vessels with higher pressures or bigger balloons,”
she said.
Honye concluded, “At the time of the first intervention,
in order to prevent restenosis you should do as much
as possible. For this purpose, IVUS, CFR, FFR and other
such diagnostic modalities should be used effectively
to obtain optimal results.” |
PAGE
TOP
|
New Clinical Approaches to Restenosis |
“Stents have been important
because of their acute ability to provide good geometry
of the vessel. Unfortunately, stents have not been the
complete solution to restenosis,” said Richard
E. Kuntz, of Brigham & Women's Hospital, Boston,
Massachusetts.
Angiographic and clinical parameters of restenosis can
be used to construct a multivariate model that predicts
restenosis. According to Kuntz, this model includes,
as the most important predictors of restenosis, post-treatment
lumen diameter, lesion length, and presence of diabetes.
“Patients with large lumens, short lesions, and
no diabetes have the lowest restenosis rates,”
he observed. “Even more important than lesion length,
however, is the length of the stent the operator implants.
For diffuse disease, the choice of a shorter stent will
reduce the restenosis rate more than the length of the
lesion. When confronted with a long lesion, spot stenting
is probably the best thing to reduce restenosis.”
Kuntz cautioned, however, that even within this model
there is great variability. Based on various patient
characteristics, the rate of restenosis in the model
ranges from 6% to 46%. “In the literature, studies
tend to report results in non-diabetic patients with
short lesions and large vessels. But in our practice,
we tend to work on patients with diabetes with longer
lesions and smaller vessels, so the restenosis rates
we see in the real world tend to be in the higher range,”
he observed. In arteries 2.5 mm or smaller, stents may
not offer an advantage over balloon angioplasty alone,
he added.
A decade of preclinical and clinical investigations
has now established radiation therapy as a valid means
of reducing restenosis. Four multicenter randomized
studies have demonstrated reductions of 35-66%.
For example, in the GAMMA 1 study, gamma radiation achieved
a 57% reduction in restenosis within the stent, and
a 41% reduction in the area immediately outside the
stent. Clinically, the patients benefited as well, with
significant reductions in major adverse coronary events,
and target lesion and vessel revascularization. “What’s
more, radiation therapy tended to work best in patients
with the highest predictors of restenosis,” Dr.
Kuntz added (those with longer lesions and diabetes).
The newer beta radiation therapy appears to be as powerful
as gamma radiation in reducing restenosis. In the START
trial of 476 patients, which evaluated the Beta Cath
system, significant reductions were demonstrated in
rates of restenosis, target vessel and lesion revascularization,
and major coronary events. This trial also proved that
the problem of late thrombosis could be ameliorated
by avoiding the use of new stents and extending antiplatelet
therapy, Dr. Kuntz reported.
In addition to the six randomized trials showing radiation
to be effective in reducing restenosis in coronary arteries,
the first randomized trial of radiation therapy in vein
grafts, presented at the American College of Cardiology
by Waxman et al, produced “spectacular outcomes,”
according to Dr. Kuntz. “Reductions in restenosis
for all measures of angiography as well as clinical
outcomes suggest that in-stent restenosis in vein grafts
has the same biology and response as in native coronaries,”
he said.
“This brings us to seven randomized trials, all
positive, and all showing reductions and prevention
of restenosis in patients receiving radiation therapy.
There is no doubt that radiation therapy is an effective
treatment for in-stent restenosis,” he concluded. |
PAGE
TOP
|
| At this time, brachytherapy
remains the only approved method of reducing restenosis.
New, alternative approaches, such as biodegradable self-expanding
coil stents, however, show promise. Other new approaches
include drug-coated stents, gene therapy, photodynamic
therapy, ultrasound therapy, and cryotherapy. Basic
science studies and animal models have yielded encouraging
results, and clinical trials of some of these new modalities
are now in progress, stated Kuntz. |
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2001
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|


