 |
|
|
 |
| Treatment of Severe Cardiac Arrhythmias |
|
Chikaya Omichi, M.D.
Cedars-Sinai Medical
Center, Los Angeles, California
Tsuyoshi Shiga, M.D.
Tokyo Women's
Medical University, Tokyo, Japan
Hideo Mitamura, M.D.
Keio University, Tokyo,
Japan
Kenzo Hirao, M.D.
Tokyo Medical and
Dental University Tokyo, Japan
Ichiro Watanabe, M.D.
Nihon University,
Japan
Koichiro Kumagai, M.D.
Fukuoka University,
Fukuoka, Japan |
|
|
|
 |
|
|
 |
|
Research from six Japanese
centers was presented in this session, after a review
of the prevention of sudden cardiac death in high-risk
patients and the use of internal cardioverter defibrillators
for primary and secondary prevention for sudden cardiac
death.
Hypertrophic cardiomyopathy (HCM), long QT syndrome,
idiopathic ventricular fibrillation, Brugada syndrome,
and arrhythmogenic right ventricular dysplasia are associated
with a high risk for sudden cardiac death (SCD) prevention.
Clinical predictors associated with increased risk of
SCD include young men with a history of syncope and
a positive family history of SCD, markers such as non-sustained
ventricular tachycardia, inducible ventricular arrhythmias
during electrophysiologic testing, ischemia during exercise
testing, and severe HCM. Hypertrophy exceeding 30 mm
of thickness is reported to have a 15% positive predictive
value. The relative risk of SCD increases as the number
of predictors increase.
ICD implantation is a class II indication for high-risk
patients according to the current guidelines, and the
value of anti-arrhythmic therapy and electrophysiologic
study is limited. The ICD has been shown to be most
effective in the primary prevention trials and its benefit
is most marked in patients with ejection fractions at
least less than 40% and probably less than 30%. For
secondary prevention, ICD implantation is a class I
indication, and drugs or ablation for frequent discharges
can be implemented, especially in patients with recurrent
monomorphic ventricular tachycardia. For patients with
severe heart failure associated with these disease syndromes,
medical therapy or heart transplantation can be considered.
Adjunctive therapy of beta blockers, aspirin, statins
and ACE inhibitors should be used routinely. |
|
Amiodarone
Infusion, Wavefront Dynamics and Ventricular Fibrillation
|
Intravenous amiodarone has
gained popularity for the emergent management of life-threatening
ventricular arrhythmias, and it has been recently reported
that treatment with amiodarone results in a higher rate
of survival to hospital admission in patients with cardiac
arrest due to refractory ventricular arrhythmia. However,
the mechanisms of the antifibrillatory action of amiodarone
are not fully understood.
Amiodarone was shown to progressively increase the cycle
length of ventricular fibrillation (VF) in a swine model
by Omichi and colleagues at Cedars-Sinai Medical Center,
Los Angeles, California. A reduction of the low amplitude
and fast activation in VF, leading to the transition
to ventricular tachycardia (VT) or a slower cycle length
of VF was demonstrated. Amiodarone reduced the density
of wavelets and suppressed spontaneous wavebreaks, and
progressively increased the cycle length of the reentrant
wavefront and the central core area. This study demonstrated
that amiodarone flattens the action potential duration
(APD) restitution curve and reduced the incidence of
spontaneous wavebreaks |
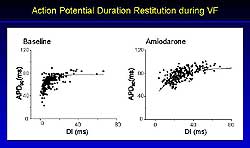 |
| Figure
2. APD restitution curves with a slope less than
1 during VF and pacing were flattened with amiodarone. |
| Click
to enlarge |
|
Amiodarone reduced
the low amplitude of fast activation seen at baseline
and also increased the cycle length of VF from 71 ms
to 103 ms. Amiodarone slowed the rate of VF in 4 of
6 cases, terminated VF in 1 case, and converted VF to
VT in 1 case. The effect of amiodarone on the transmembrane
action potential is shown in Figure 1. Amiodarone significantly
increased the mean cycle length (CL) of VF from 80 to
110 ms (p<0.05 versus baseline). APD90 was significantly
increased from 70 ms to 90 ms after amiodarone. The
diastolic interval increased progressively from 12 ms
to 20 ms after amiodarone infusion.
Amiodarone reduced the density of the wavelets from
0.65 ± 0.08/cm2 at baseline to 0.41
± 0.10/cm2 (p<0.05), and significantly
reduced the number of wavelengths from 35/second to
11/second (p<0.05). The cycle length of the reentrant
wavefront increased significantly from 78.2 ±
19.0 ms at baseline to 108.9 ± 13.3 ms after
amiodarone infusion in each case (p<0.05), and the
central core area increased from 0.9 ± 0.3 mm2
to 4.1 ± 3.8 mm2 after amiodarone
(p<0.01). Amiodarone flattened the AP90
restitution curves with a slope less than
1 during VF and pacing (Figure 2). The slope maximum
of the AP90 restitution curves in each case
was significantly decreased from 2.2 to 1.2 after amiodarone
(p<0.05). |
PAGE
TOP
|
Therapeutic
approaches to VF |
A retrospective study of VF in patients with or without
structural heart disease (SHD) was undertaken due to
the lack of therapeutic guidelines in Japan for the
long-term management of survivors of VF and SCD. Combination
therapy of ICD and drug therapy may prevent SCD, heart
failure death, and total cardiac death in patients with
underlying SHD, concluded Tsuyoshi Shiga, MD, Tokyo
Women's Medical University. ICD therapy alone can
prevent SCD and total cardiac death in patients without
SHD and idiopathic VF. Beta blockers as an adjunctive
therapy with ICD can reduce the excessive number of
ICD discharges.
Despite compelling uncontrolled data, controlled data
from clinical trials are needed to support the observed
effects of beta blockers in this retrospective study,
stated Shiga. Amiodarone seems to have antifibrillatory
effects in VF and powerfully suppresses premature ventricular
contractions. It also has a non-competitive anti-adrenergic
action, thus beta blocker-type side effect can be avoided.
Amiodarone increases the wavelength.
In the 33 cases of idiopathic VF without SHD (23 Brugada
syndrome, mean age 43 years, 28 male) no deaths were
observed after ICD implantation. At 1 and 5 years, 30%
and 50% of patients had an ICD discharge, respectively.
The ICD discharge rate was higher in the patients with
Brugada syndrome compared to patients without, although
the number of Brugada patients was small. In the Brugada
patients with inducible VF, 3 of the 5 had ICD discharge,
while those without inducible VF had no ICD discharge.
In the 33 cases with SHD, the ICD discharge rate was
the same as in the patients without SHD. No SCD occurred
in patients with an ICD, but the total cardiac mortality
was 7% at 1 year and 37% at 6 years. Thus, ICD seems
to prevent SCD, but not total cardiac death. Ten of
the patients had CAD, with a higher percentage being
non-ischemic (hypertrophic or idiopathic dilated cardiomyopathy),
which may be unique to the Japanese population, and
the mean age was 50 years and 23 were male.
Of the 20 patients treated with an ICD, 3 patients died
from heart failure death, but not SCD. In 12 patients
treated with amiodarone alone, 4 patients died from
SCD; no heart failure deaths. In the patients treated
with an ICD, 3 of the 9 patients died, while none of
the 12 patients treated with an ICD and drug therapy
died. The left ventricular ejection fraction was less
than 30% in the 3 patients who died. |
PAGE
TOP
|
Electrical
remodeling and selection of antiarrhythmic drugs |
Pharmacologic conversion
of (AF) can be difficult, particularly when the AF has
been sustained for weeks. It has been reported that
prolonged atrial tachyarrhythmia causes intracellular
calcium overload, leading to electrical remodeling characterized
by changes in structure and function of ion channels.
Thus, it is reasonable to consider that the antiarrhythmic
efficacy of drugs affecting these channels or receptors
may also change.
The serial changes of electrophysiologic (EP) effects
of three different drugs that block the Ina (pilsicainide),
Ikr (E4031), and IKs (azimilide) channels, respectively,
were studied in a canine model of rapid atrial tachycardia.
The study conducted by Mitamura and colleagues at Keio
University in Tokyo then evaluated whether a calcium
channel blocker can prevent the development of atrial
electrical remodeling in the same model. |
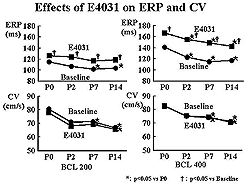 |
| Figure
3. The effective refractory period is increased
with the IKr blocker E4031. |
| Click
to enlarge |
|
The effective
refractory period (ERP) became progressively shorter
over the 14-day period of rapid atrial pacing, indicating
electrical remodeling. In the pilsicainide group (0.6mg/kg
loading + 0.04 mg/kg/min maintenance), the ERP was prolonged,
the conduction velocity (CV) decreased, and the wavelength
(WL) increased significantly at baseline, but these
effects dissipated after 2 days of rapid pacing. In
the E4031 group (30 mcg/kg/3 min + 0.1 mcg/kg/min) the
ERP was increased, particularly when the pacing cycle
length was 400 compared to 200 (Figure 3). This drug
prolonged ERP in a reverse use-dependent fashion. The
effect of prolonging ERP was unaffected by the duration
of rapid atrial pacing. No effect on CV was seen with
this drug, as expected.
The WL is a product of ERP and CV, and the difference
between pilsicainide and E4031 was easily apparent.
With pilsicainide, the WV was only prolonged at p0,
before the initiation of rapid atrial pacing, but rapid
atrial pacing did not prolong the WL. In contrast, E4031
increased the WL throughout the study period, which
was more marked when the cycle length was 400. |
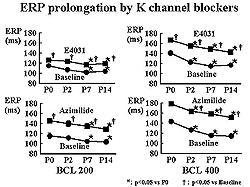 |
| Figure
4. The IKs blocker azimilide prolonged the effective
refractory period. |
| Click
to enlarge |
|
Pilsicainide
failed to suppress the induction of AF in all dogs in
the group, whereas E4031 suppressed the induction of
AF in 1 of 3 inducible dogs. Azimilide, a combined IKr
and IKs blocker, prevented the induction of AF. Azimilide
does not have a reverse use-dependent pattern, like
E4031, as shown by its ability to prolong ERP at both
200 and 400 cycle length (Figure 4).
The EP effects of sodium channel blockers waned as rapid
atrial pacing persisted, while the EF effects of potassium
channel blockers were well preserved. Since a pure Ikr
blocker showed a reverse use-dependent effect, only
a combined Ikr plus Iks blocker was effective in preventing
AF inducibility in the electrically-remodeled canine
atrium. |
Treatment with a calcium blocker prevented the development
of pacing-induced atrial electrical remodeling in the
canine atrium. Verapamil (oral 8 mg/kg/day) was begun
1 week prior to rapid atrial pacing in 8 dogs. In the
12 control dogs, the ERP decreased over the 14 days
of rapid atrial pacing, whereas in the verapamil-treated
dogs the ERP was longer than in the control dogs and
did not change over the 14 days. CV was not decreased
in the verapamil-treated dogs. AF was induced after
7 days of rapid atrial pacing in the control dogs, whereas
the duration of induced AF was shortened in the verapamil-treated
dogs.
This study showed that drugs blocking ion channels with
downregulation, such as sodium channel blockers, may
lose their efficacy, stated Mitamura. The efficacy of
other drugs, such as IKr and IKs blockers, may be preserved.
Prevention of calcium overload by calcium blockers can
prevent atrial electrical remodeling. Understanding
these concepts will help to select appropriate drugs
for the management of persistent atrial tachyarrhythmias. |
PAGE
TOP
|
Relationship
between Ic-Atrial Flutter and Therapeutic Drug Monitoring
|
Atrial flutter (AFL) can
be documented after the initiation of treatment with
several antiarrhythmic drugs in patients with atrial
fibrillation (AF). In class 1c-AFL patients, combination
therapy with catheter ablation of the right atrial isthmus
and continued drug therapy (hybrid therapy) is reported
to be a promising means to achieve and maintain sinus
rhythm (SR). A prospective study conducted by Hirao
and colleagues at Tokyo Medical and Dental University
showed that AF could be converted into AFL with the
class 1c drug, pilsicainide, especially with a high
dose. Catheter ablation of class 1c-AFL and persistent
pilsicainide was effective in reducing AF. Further and
larger trials with this therapy including the intravenous
administration of a class 1c drug is needed to elucidate
the real benefit of hybrid therapy for AF, stated Hirao.
In Group 1 (83 patients with AF, 70 paroxysmal or persistent),
pilsicainide caused paroxysmal or persistent AFL in
12 patients. Catheter ablation was performed in 10 of
the 12 class 1c-AFL patients; 2 patients refused. In
Group 2 (10 patients with AF and AFL), 7 patients had
catheter ablation after pilsicainide treatment.
Catheter ablation for isthmus-dependent AFL was successful
in 12 patients. In 11 patients who continued to receive
pilsicainide after successful catheter ablation, SR
persisted in 6 patients. In the 5 patients with recurrent
AF, pilsicainide was changed to another class I drug,
which maintained SR. In 1 patient who preferred not
to have any antiarrhythmic agent, SR rhythm has been
maintained. |
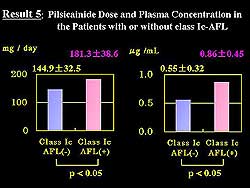 |
| Figure
5. The dose and plasma concentration of pilsicainide
in patients with and without class Ic-atrial flutter. |
| Click
to enlarge |
|
The dose of
pilsicainide associated with conversion of AF to 1c-AFL
was significantly higher than the dose not associated
with this conversion (181.3 ± 38.6 mg/day vs
144.9 ± 32.5 mg/d, respectively; p<0.05) (Figure
5). The plasma concentration was also significantly
higher in the patients converted to 1c-AFL than those
who did not (0.86 mcg/mL ± 0.45 vs 0.55 mcg/mL
± 0.32 mcg/mL; p<0.05). The duration of AF
was 28 months in the patients with inducible Class 1c
AFL, 49 months in the AF patients, and 19 months in
the patients with suppressed AF and maintenance of SR
(p<0.05).
The incidence of 1c-AFL was 6.1% in the patients who
maintained the initial dose of pilsicainide and 47.1%
in the patients in whom the dose was increased. The
overall incidence of 1c-AFL was 14.5%. Permanent AF
or AF with a long history tended to convert to 1c-AFL
less frequently. Successful catheter ablation of 1c-AFL
with continued pilsicainide was effective in 4 patients
and partially effective in 4 patients. Limitations to
this study noted by Hirao include the fact that short-lasting
paroxysmal or non-symptomatic episodes of 1c-AFL may
not be recorded, and drug complications, especially
the risk for 1c-AFL with 1:1 AV conduction. |
PAGE
TOP
|
Low-Energy
Internal Cardioversion of Chronic AF |
Early recurrence of AF is
common after cardioversion of chronic AF. The currently
used clinical parameters of the duration of the antecedent
episode and the size of the left atrium on echocardiogram
are weak predictors of outcome. Frequency analysis of
the ECG and signal-averaging ECG of the P-wave duration
(P-SAE) after cardioversion was prospectively applied
to predict the recurrence of AF after internal atrial
cardioversion (IACV) in 22 patients with chronic, non-valvular
AF (mean age 63 years, 15 males, mean AF duration 21
months).
IACV was successful in all 22 patients, with a mean
energy of 8.6 J. Nine of the 22 patients who completed
the 3-month follow-up had documented recurrence of AF,
stated Ichiro Watanabe, MD, Nihon University. Neither
clinical nor echocardiographic variables predicted the
recurrence. The peak frequency of AF is higher and the
filtered P wave duration (FPD) derived from the P-SAE
was longer in patients who went on to early recurrence
after IACV than in the patients who remained in SR.
The peak frequency of AF and the FPD derived from P-SAE
better predict outcome than clinical or echocardiographic
variables and may be useful for the management of patients
with AF, stated Watanabe.
The average peak frequency of AF was 7.36 ± 0.44
Hz in recurrent cases, compared to 6.65 ± 0.33
Hz non-recurrent cases (p<0.001), establishing a
cut-off of 7 Hz. For a peak frequency greater than 7
Hz, there were 7 recurrent cases and 2 non-recurrent
cases. For a peak frequency less than 7 Hz, there were
2 recurrent cases and 10 non-recurrent cases (p<0.001).
The average filtered P wave duration (FPD) was 161.5
± 10.6 ms for recurrent cases and 151.2 ±
19.6 ms for non-recurrent cases (p<0.05). For an
FPD greater than 145 ms, there were 8 recurrent cases
and 6 non-recurrent cases, and for an FPD less than
145 ms there were no recurrent cases and 7 non-recurrent
cases (p<0.02). No statistical difference was found
for the clinical and echocardiographic parameters of
age, AF duration, left ventricular ejection fraction
and left atrium diameter.
IACV was successful in all 22 patients, with a mean
energy of 8.6 J. Nine of the 22 patients who completed
the 3-month follow-up had documented recurrence of AF.
Neither clinical nor echocardiographic variables predicted
the recurrence. The peak frequency of AF is higher and
the FPD derived from the P-SAE was longer in patients
who go on to early recurrence after IACV than in the
patients who remained in SR. The peak frequency of AF
and the FPD derived from P-SAE better predict outcome
than clinical or echocardiographic variables and may
be useful for the management of patients with AF. |
PAGE
TOP
|
Pulmonary
Venous Foci-Initiated AF |
|
|
Kumagai and
colleagues showed in a prospective study that the focal
sources initiating AF usually are located in the pulmonary
vein (PV), and careful mapping and elimination of these
foci and atrio-PV conduction can cure AF. Therefore,
combination therapy of focal ablation and PV isolation
is more effective that either strategy alone, stated
Kumagai. .
Mapping was performed in 60 patients (age 60 years,
45 males) during spontaneous atrial premature beats
(APB) or AF initiation with isoproterenol infusion or
APB after cardioversion. A PV confirmed by the earliest
atrial activation was targeted for ablation. The elimination
of AF was the defined endpoint. If patients had no APB
or AF initiations, all APB displaying distinct and late
PV potential during sinus rhythm were targeted for PV
isolation. The endpoint was determined by abolition
of PV potentials and/or creating atrial PV conduction
blocks. After identification of the PV site, the basket
catheter was positioned within the PV (Figure 6). |
| Of the 104 foci identified, 90% originated from the
PV and 10% originated from the atrial tissue; 47% of
the patients had one focus, 31% two foci, and 22% had
3-4 foci. The first ablation was successful in 28 patients.
In the 17 patients who underwent a second ablation,
the recurrence was from the same source in 7 patients,
from a different part of the same PV in 3, from a different
PV in 2, and the PV potentials and A-PV conduction in
5 patients. Pericardial effusion occurred in 2 patients
after successful ablation but no PV stenosis was observed.
The success rate for ablation was 81% for one focus,
56% for two foci, and 44% for 3-4 foci. The success
rate for paroxysmal AF was 73%, 46% persistent AF, and
40% permanent AF. The success rate for focal ablation
only and for PV isolation ablation only was 60%, and
75% for combined focal ablation and PV isolation. |
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2001
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|