 |
|
|
 |
| New Strategies in the Surgical Management of Advanced Heart Failure
|
|
Hisayoshi Suma
Hayama Heart Center,
Miura, Japan
Takuya Nomoto
Kyoto University Graduate
School of Medicine, Kyoto, Japan
Goro Matsumiya
Osaka University Graduate
School of Medicine, Osaka, Japan |
|
|
|
 |
|
|
 |
|
Left Ventriculoplasty for Cardiomyopathy |
|
Left ventriculoplasty is a vital option for the treatment
of end-stage cardiomyopathy, according to results
of a study presented by Hisayoshi Suma. He and his
colleagues at the Hayama Heart Center have performed
left ventriculoplasty in 238 patients over the last
5 years, including 138 patients with left ventricular
(LV) dysfunction caused by coronary artery disease
(CAD) and 100 patients with non-ischemic cardiomyopathy,
mostly idiopathic dilated cardiomyopathy.
Among the 86 patients with ischemic cardiomyopathy,
the average patient had multivessel disease and a
preoperative ejection fraction of 22.8%. All patients
had significant heart failure in spite of appropriate
medical treatment, though angina pectoris was uncommon.
The LV dilatation was prominent, with elevated pulmonary
wedge pressure. The Dor procedure was performed on
70 patients, and the remaining patients underwent
a new procedure of septal anterior ventricular exclusion,
termed the SAVE operation. The Batista operation was
used in only 4 patients with ischemic cardiomyopathy.
Concomitant coronary artery bypass grafting was done
in 93% of patients, mitral valve reconstruction in
42%, and tricuspid repair in 15%--indicating the extent
of ventricular dilation, with valvular insufficiency
in addition to severe CAD.
In this group, the overall hospital mortality was
11.6%, although it was only 5.4% for the 74 elective
procedures, rising to 50% among patients who required
emergency surgery. There were 7 late deaths, mostly
due to heart failure or sudden death. The procedure
raised the mean ejection fraction from 23% to 36%,
and reduced LV diameter and volume as well as pulmonary
wedge pressure. The 4-year survival was 76.4%.
Among 73 patients with idiopathic non-ischemic dilated
cardiomyopathy treated with ventriculoplasty, 61 underwent
the Batista operation and the other 12 were treated
with the new SAVE procedure, which excludes the akinetic
septum. Mitral valve reconstruction was done concomitantly
in all but one patient, and tricuspid repair was added
in 66% of the patients.
Hospital mortality in this group was 20.5%, with
an 8.9% rate for elective operations and 58.8% for
emergency surgery. The procedure raised the mean ejection
fraction from 21% to 31%, and reduced LV diameter
and volume and pulmonary artery pressure. Brain natriuretic
peptide decreased steadily from 1125 before the operation
to 716 at 1 month and 427 at 6 months.
Describing the lessons learned from doing the Batista
operation in these patients, Dr. Suma noted that the
extent of LV fibrosis is not uniform. When fibrosis
is greater in the septum than the lateral wall, the
Batista operation is theoretically unsuitable. Mortality
reached 55% in this group with "bad septums,"
therefore, the new SAVE procedure is recommended,
which excludes the septum instead of excising the
lateral wall. Expanding on patient selection, when
the Batista operation was applied without any selection
criteria, the in-hospital mortality rate was 43%,
but this decreased to 15% with the use of site selection
and intraoperative echo evaluation, which helps guide
the choice of procedure. In the most recent 47 patients
undergoing elective surgery, the hospital mortality
was only 6.4%.
|
PAGE
TOP
|
Adjuvant Therapies for Left Ventricular Aneurysm |
|
Adjuvant therapies may improve outcomes after LV
repair for LV aneurysm. Takuya Nomoto and colleagues
found that angiotensin converting enzyme inhibitors
(ACEI) and cardiomyocyte transplantation were useful
for preventing re-dilation and maintaining LV systolic
function, possibly extending the benefit of surgery
for this condition.
ACEIs are well known means of attenuating post-infarction
LV remodeling. In this study, ACEI after LV repair
attenuated LV remodeling and maintained LV function,
which was associated with lower oxidative stress.
The study was conducted in rats with LV aneurysm who
were divided into three treatment groups: (1) sham
operation plus ACEI therapy (group A); (2) LV repair
and placebo (group R); (3) LV repair and ACEI (group
RA). The drug was administered for 4 weeks, after
which the animals were evaluated and sacrificed.
|
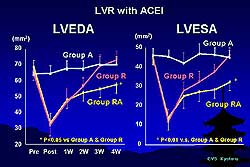 |
| Figure
1. LV remodeling was prevented and LV end-diastolic
area (LVEDA) was significantly smaller in the
group receiving both active treatments (LV repair
and ACE inhibitor, group RA), compared to the
groups receiving a sham operation plus ACEI therapy
(group A) and LV repair and placebo (group R).
|
| Click
to enlarge |
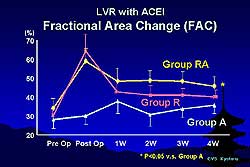 |
| Figure
2. Fractional area change (FAC), an estimate of
LV systolic function, remained steady for group
A but dramatically increased in the two LV repair
groups, achieving the highest levels in the ACEI-treated
animals. |
| Click
to enlarge |
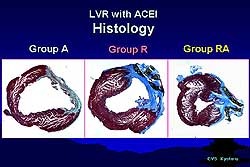 |
| Figure
3. Histological sampling showing a large infarction
in group A and severe fibrosis in group R, but
only slight fibrosis and smaller LV size in the
LV repair/ACEI group. |
| Click
to enlarge |
 |
| Figure
4. The effect of LVR alone and LVR/CM-TX on left
ventricular end diastolic and systolic pressures. |
| Click
to enlarge |
|
In the animals receiving both active treatments (group
RA), LV remodeling was prevented and LV end-diastolic
area (LVEDA) was significantly smaller than in the
other two treatment groups (Figure 1). Rats with the
sham operation and the ACEI therapy (group A) maintained
almost the same LVEDA throughout the study. In the
rats undergoing LV repair without ACEI (group R),
the area decreased after LV repair but gradually re-dilated.
Results were similar for the LV end-systolic area
(LVESA).
In animals receiving ACEI, both LV and right ventricular
weights were lower. Also in these groups, immunohistochemical
staining showed oxidative stress (by 8-hydroxy-2'-deoxyguanosine,
a marker) to be much lower than in animals receiving
placebo. Fractional area change (FAC), which estimates
LV systolic function, remained steady for group A
but dramatically increased in the two LV repair groups,
achieving the highest levels in the ACEI-treated animals
(Figure 2). Histological sampling showed a large infarction
in group A and severe fibrosis in group R, but only
slight fibrosis and smaller LV size in the LV repair/ACEI
group (Figure 3). Also in the combination treatment
group, brain natriuretic peptide mRNA in areas near
and remote from the aneurysm was reduced.
In the second study, the combination of cardiomyocyte
transplantation (CM-TX) after LV repair also proved
beneficial to outcome by attenuating LV remodeling
and maintaining LV function. Rats with LV aneurysm
were divided into three groups: (1) cell transplantation
group; (2) LV repair group; (3) LV repair plus cell
transplantation. Echocardiography revealed that at
4 weeks LV size remained unchanged in the CM-TX group;
decreased but then redilated in the LV repair group;
but decreased and remained smallest in the LV repair/CM-TX
group. Also in this group, FAC was highest, LV end-diastolic
pressure was lowest, and E-max was highest. In the
LV repair group, fibrosis developed and LV size was
large, while in the combination therapy group fibrosis
was slight and transplanted cardiomyocytes were detected
in the peri-infarct area. Figure 4 shows the effect
of LVR alone and LVR/CM-TX on left ventricular end
diastolic and systolic pressures.
Dr. Nomoto concluded that adjuvant therapies helped
prevent postoperative LV remodeling and may make LV
repair a more effective surgical treatment for LV
aneurysm.
|
PAGE
TOP
|
Left Ventricular Assist System for End-Stage Heart Failure |
|
A left ventricular assist system (LVAS) has become
an important modality for end-stage chronic heart
failure, not only as a bridge to transplant but also
as a permanent support or a bridge-to-recovery procedure.
Goro Matsumiya presented the data from his center
on 42 implantations of LVAS since 1992, including
18 implantable and 24 extracorporeal LVAS devices.
The majority of patients (mean age 41) had idiopathic
dilated cardiomyopathy.
The implantable LVAS devices clearly provided a better
quality of life and a survival benefit over the extracorporeal
devices. Over 50% of patients were alive at 1 year
with implantable devices, compared to only 20% with
the extracorporeal LVAS (p < 0.05). Patients who
underwent procedures after 2000 had improved survival
over the earlier recipients.
Among the 18 patients with implantable LVAS (7 with
Novacor, 11 with HeartMate), 3 were successfully bridged
to heart transplantation and 6 are awaiting transplant;
9 patients have died, 6 of them from infectious complications.
There was 1 transplantation among the 24 extracorporeal
device patients. While awaiting organs, the 4 transplanted
patients were supported by LVAS for up to 3 years.
Eight patients with the implantable LVAS have been
followed for over 6 months and 5 patients for over
1 year. Complications have included infection, primarily
serious pump pocket infection and gastric ulcer and
perforation in patients of small body size. These
complications were successfully managed, and there
were no deaths among patients who survived more than
6 months after implantation.
Histological analyses demonstrated an increase in
percent fibrosis of the left ventricle after the procedure
(from 20-30% to 50-60%) and an increase in apoptosis.
For the 3 patients with the implantable device who
received heart transplants, 2 had the dilated phase
of hypertrophic cardiomyopathy, which is known to
progress rapidly to severe fibrosis. These patients
had 20-30% fibrosis at the time of LVAS implant, which
progressed to over 60% by the time of transplant.
One patient had dilated cardiomyopathy and 51% fibrosis
at the time of LVAS implantation, progressing to 60%
at transplant, after which she functionally improved.
For both implantable and extracorporeal devices,
percent fibrosis at baseline was significantly correlated
with LV function post-procedure: patients with less
severe fibrosis had a better recovery of function
than patients with severe fibrosis (> 40%), who
had no meaningful functional recovery.
In sum, functional recovery was observed with implantable
LVAS support in some patients with cardiomyopathy,
especially those with the least fibrotic myocardial
damage. The optimal use of LVAS may be in patients
with sufficient residual myocardial viability with
mild fibrosis.
|
|
In other studies from Dr. Matsumiya's laboratory,
gene transfection with hypertrophic growth factor
(HGF), which potentially has anti-fibrotic, anti-apoptotic
and mitotic activity, improved cardiac function in
a canine model of dilated cardiomyopathy. Direct administration
of the gene into the myocardium significantly increased
left ventricle wall thickness and myocyte diameter
and improved cardiac function. Going a step further,
they combined HGF gene transfection with myocyte transplantation
in an LVAS-supported heart failure LAD ligation model
to enhance blood supply and attachment of grafted
cells. The combined therapy was superior to cell transplantation
alone in preserving LV function and anterior wall
motion and enhancing myocardial perfusion. Finally,
the investigators are working on tissue-engineered
cardiomyocyte grafts, showing that at 2 weeks post-grafting
the cardiac seeds resemble cardiac tissue, adhere
to the surface of the ventricle, and even migrate
into native heart tissue, improving global cardiac
function.
These investigators have concluded that regeneration
therapy, including gene therapy using HGF, myocyte
transplantation, and tissue-engineered cardiac grafts,
are promising strategies that will promote recovery
of cardiac dysfunction, including patients on LVAS.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2002
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|