|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Optimal Timing of Operation for Aortic RegurgitationSatoshi NakataniNational Cardiovascular Center
|
||||||||||||||||||||||||||||||||
|
Data from Sarano and colleagues at the Mayo Clinic show the clinical events in medically-managed severe aortic regurgitation (AR). Over 10 years, about 83% of patients with significant AR have cardiovascular events, including cardiac death, surgery (62%), congestive heart failure (47%), vascular complications (15%), atrial fibrillation, and endocarditis.
The American College of Cardiology-American Heart Association (ACC-AHA) guidelines for the management of severe AR is shown in Figure 1. For symptomatic patients, the guidelines recommend aortic valve replacement (AVR). For asymptomatic patients, the guidelines state:
- Assess ejection fraction (EF) to guide treatment.
- For a normal EF, assess diastolic or systolic diameter.
- Diastolic diameter > 75 mm or systolic diameter > 55 mm indicates AVR.
The indications for surgery in patients with asymptomatic severe AR, based on the European Society of Cardiology (ESC) guidelines are:
- Left ventricular (LV) end diastolic diameter > 70 mm.
- LV end systolic diameter > 55 mm.
- End systolic diameter index > 25 mm.
- Ascending aortic dilatation >55 mm.
- Rapid increase in LV diameters.
- Bicuspid atrial valve or Marfan syndrome with aortic diameter >50 mm.
The use of an LV end systolic diameter (LVESD) > 55 mm is based on a paper by Bonow published in 1982 in which patients were divided into 2 groups based on whether there LVESD is more or less than 55 mm. Patients with an LVESD > 55 mm had a poor prognosis.
LVESD > 55 MM: AVR too late?
However, whether or not this was the best criterion was questioned at that time. Fioretti and colleagues questioned in a paper published in 1983 whether AVR was too late when the LVESD was already > 55 mm. In their comparison of patients, divided into 2 groups based on a LVESD more or less than 55 mm, they showed that both New York Heart Association (NYHA) functional class and LV end diastolic diameter (LVEDD) improved in both groups after AVR. They authors stated in their discussion that, “in aortic insufficiency, a preoperative ESD greater than or equal to 55 mm does not preclude successful AVR”
|
Dujardin and colleagues from the Mayo Clinic showed that patients with an LVESD> 25 mm, compared to < 25 mm, had a poor prognosis.
Nakatani questioned whether it is appropriate to apply to Japanese patients the same equation for determining the ratio of LVESD to body surface area that is used in Western Caucasian patients. Nakatani showed that a LVESD >45.25 mm is a more appropriate indicator for AVR in male Japanese patients (Figure 2).
Heart failure after AVR
Tornos and colleagues studied 87 patients who underwent AVR and found 19 patients developed heart failure over a mean 6 year follow-up, and 14 patients died during this time period. The incidence of heart failure at 5 years was 16% and 24% at 10 years. The predictors of heart failure after AVR include age > 50 years, systolic diameter > 50 mm, and EF < 40%. Tornos and colleagues also showed that when comparing the uncomplicated patient group and the complicated/death patient group, only small differences in the LV diastolic (1 mm) and systolic (3 mm) diameters and EF (5%) before AVR resulted in large differences in surgical outcomes.
|
||||||||||||||||||||||||
The probability of developing heart failure after AVR, based on data from Tornos and colleagues, is 10% for an EF of 50% and 20% for end systolic diameter of 55 mm.
Therefore, based on all these data, Nakatani believes that AVR should be performed before the LVESD reaches 50 mm.
Other indices for AVR
Borer and colleagues developed a load-adjusted contractility index that considers the change in EF and the end systolic stress index to predict cardiac events after AVR. This index predicts patient outcomes well.
A retrospective study by Nakatani and colleagues of 97 patients who underwent successful AVR showed that pre-operatively 64 patients had an LVESD < 50 mm (small LVESD) and 33 patients had an LVESD ≥50 mm (large LVESD). After AVR, 5 patients with a large pre-op LVESD and 5 patients with a small pre-op LVESD had a final LVESD > 40 mm. When dividing the patients based on post-operative values, they found that patients with an LVEDD >60 mm and LVESD < 40 mm had poor outcomes, compared to patients with an LVEDD < 60 mm and LVESD > 40 mm. Also, 6 patients in the former group had redilatation of the ventricle in the chronic stage after surgery. Based on these results, LV size was not always reduced after AVR, even in patients with pre-op LVESD < 50 mm.
A comparison of the relative wall thickness showed that it tended to be thicker in the patients with an LVEDD >60 mm and LVESD < 40 mm compared to patients with an LVEDD < 60 mm and LVESD > 40 mm. LV mass index also tended to be greater in the former group compared to the latter group.
It has been shown that patients with hypertension and concentric LV hypertrophy have a higher rate of mortality and cardiovascular events. A review of data by Nakatani and colleagues showed that the patients with poor outcomes after AVR primarily have LV concentric or eccentric hypertrophy. Thus, pre-operative concentric or eccentric hypertrophy may relate to unsuccessful LV reverse remodeling, possibly due to myocardial damage, represented as inadequate hypertrophy.
BNP was also found to be a significant predictor of poor outcomes in patients with AVR by Nakatani and colleagues. BNP correlated very well with NYHA functional class, and notably, pre-operative BNP levels could predict the post-operative NYHA class.
Conclusions
The guidelines for the treatment of AR may need to be reconsidered. Since the 1980s, surgical outcomes have been steadily improving. Also, even in patients with a pre-op LVESD < 50 mm, some do not have normal LV function after AVR. Further, other parameters from recent data must be considered, including load-adjusted LV contractility, BNP, and types of LV hypertrophy.
Asymptomatic Mitral Regurgitation: Natural History ManagementMaurice E. SaranoMayo Clinic
|
Surgery should be considered for asymptomatic mitral regurgitation (AMR), because the evidence clearly shows that the conventional wisdom that patients with AMR do well for many years is not correct.
MR due to flail leaflet
A significant excess mortality of 43% was found in patients with MR due to flail leaflet, in a series published in the NEJM of patients who were censored when they underwent surgery; expected mortality was 35% (p=0.016). Morbidity is also high in this population. At 10 years after diagnosis, 63% have heart failure, 82% have surgery, and 90% have surgery or have died. Thus, for all practical purposes, surgery is unavoidable.
The annual rate of sudden death is 1.8% in patients in this population. In NYHA Class III-IV, the annual rate for sudden death is 7.8%, and 3.1% in NYHA Class II, and 1% in Class I. However, when looking at the number of events, only a small minority occurred in patients with frank symptoms, whereas the vast majority occurred in patients with no or minimal symptoms. Data from this series showed there were 6 events in Class III-IV, 9 events in Class II, and 10 events in Class I. In fact, the rate of sudden death with no risk factors was 0.8% annually, which is 2-times what is expected in the general population, according to Framingham Heart Study data. The risk of sudden death over 1 year is approximately equivalent to the risk of surgery in asymptomatic patients.
Surgery outcomes in AMR
MR is not a benign condition, and is associated with excess mortality and high morbidity.
Is surgery a reasonable option? The conventional wisdom is that surgery is too risky in AMR patients.
A 5.4% operative mortality was found in patients with Class III-IV, in data from Sarano and colleagues. In patients over 75 years of age with Class III-IV symptoms, the operative mortality was 12.7% and 2.5% for patients under 75 years. In Class I-II, the operative mortality was 0.5% overall, and 3.6% in the patients below 75 years. Notably, surgery the operative risk is multiplied by about 10 (from 0.5% to 5%) if a patient moves from Class I-II to Class III-IV before surgery is performed. Therefore, to wait to for rescue surgery for AMR is not a benign decision. Although it is possible to perform the surgery in that setting, it is less safe for the patient.
Long-term post-operative survival in patients in Class III-IV who became asymptomatic, still have an excess mortality. As if the fact that having symptoms with MR is associated with persistent consequences even after a successful operation.
Conversely, when operating in patients with Class I-II, the outcomes are not that different between the expected and the observed. At this stage, when surgery is performed, life expectancy has been restored to what is expected, that is, the long-term outcomes in patients with Class I-II who have surgery to what is seen in the general population. So, it is not only reasonable to perform surgery who are asymptomatic, in fact, it is the desirable option, because the goal is to restore to the patient usual life expectancy.
The conventional wisdom is that a predictable MVR is preferable to an uncertain repair. The reality is that mitral repair has transformed clinical practice.
A series by Sarano and colleagues published several years ago, shows that the survival with repair is far superior to that of valve replacement (nearly 68% vs 52%, respectively; p=0.0004).
|
Survival up to 15 years after surgery for a prolapsed mitral valve prolapse (PL-MVP) or an anterior leaflet MVP (AL-MVP) is superior for repair than for replacement (Figure 1). At 10 years, survival after AL-MVP is 63.5% and 31%, respectively for repair and replacement, and at 15 years it was 42% and 31%. For PL-MVP, 10-year survival was 70% and 49% for repair and replacement respectively, and 41% and 31% at 15 years.
Reoperation rates for surgery for MR for MVP was 24% and for AL-MVP was 10% at 10 years in the 1980s, which was reduced to 10% and 5% respectively, in the 1990s, because of the improvement in surgical techniques. This now makes it possible to perform surgery in patients with asymptomatic patients with MVR, even for ALP.
Data from surgical repair has shown that valve repair can be done consistently, at low-risk, and with great long-term results.
Patient selection
The ACC guidelines recommend using left ventricular (LV) function for patient selection. Their definition for overt LV dysfunction is an ejection fraction (EF) <60% and an end-systolic diameter >45 mm.
Data from South Africa in young patients with rheumatic heart disease
for the cumulative probability of LV dysfunction showed there is
no risk of postoperative mortality up until 45 mm end systolic diameter,
and thereafter the risk increases markedly. At Mayo, in about 650
patients who had pre- and post-operative echocardiography, Sarano
and colleagues found that at about 45 mm end systolic diameter 70%
of LV dysfunction had already occurred. Thus, the risk of surgery
is increased by delaying it until the end systolic diameter is 45
mm. Even at 40 mm end systolic diameter, already 50% of the dysfunction
has occurred. The data from Mayo was in older patients with mostly
degenerated MR, showing the need to perform surgery earlier in the
older patients.
Rescue surgery is possible in patients with a low EF < 60, a
level at which 10-year survival has been shown to be about only
53%. However, survival is 73% in patients with an LVEF >60%. Thus,
another strategy is needed in patients with normal LV function,
using the degree of MVP for patient selection.
In the Olmstead County population of patients with mitral valve prolapse, the data show that the absence of mitral regurgitation is associated with an 89% survival rate, while slight mitral regurgitation is associated with 82%, but only a 53% survival in patients with moderate or worse mitral regurgitation (p<0.001 vs expected survival). There is a gradient of events and cardiovascular morbidity increases markedly with the degree of mitral regurgitation (89% for moderate or worse MR, 22% for slight MR, and 6% for no MR). Therefore, the degree of MR is predictive of outcomes, and patients with mild MR regardless of the presence of myxomatous disease. Patients with severe MR have poor outcomes even if they are asymptomatic.
Quantification of MR
|
Color-flow estimation of MR may estimate poorly the degree of MR. The PISA (Proximal Isovelocity Surface Area) method is most commonly used today for quantitation.
In effective regurgitant orifice (ERO) calculation, the flow measurement is done on the proximal flow convergence, the contour of the left ventricular velocity of the MR jet velocity is traced, and the ratio of the flow divided by the velocity is the ERO (Figure 2).
The grading of the severity of MR in the ACC guidelines is shown in Figure 3. A regurgitant volume >60 mL or a regurgitant orifice >40 mm2 is severe mitral regurgitation in terms of the amount of regurgitation. But, is it severe for outcomes? In fact, Sarano and colleagues recently published in the New England Journal of Medicine results from a 15-year follow-up that included the first patient in 1991 in whom they performed quantitation of MR. In the 456 patients enrolled prospectively over 10 years with an EF >50%, asymptomatic, and managed by their independent physician. The mean age was 64 years (the standard for MR) and 38% were women, and the mean regurgitant volume 68 mL and the mean ERO 43 mm2. Survival at 5 years was highly and independently related to the ERO (Figure 4). An ERO of 1-19 mm2 was associated with a 91% survival, an ERO of 20-39 mm2 associated with a 66%, and an ERO >40 mm2 was only 58% (p=0.03 vs expected).
In this study, the predictors of outcome, including survival, were age, diabetes, and ERO. An ERO >40 mm2 was associated with a 2.9 times increased risk for total mortality, cardiac mortality 5.2 times increased risk, and cardiac events 5.7 times increased risk. Clearly, compared to expected survival, patients with an ERO >40 mm2 incur excess mortality.
In this study, 232 patients had surgery (2 bypass surgery, 209 valve repairs, 21 valve replacements) and the operative mortality was 0.9%. Adjusting for ERO in these patients, surgery markedly reduced mortality (RR 0.28) and markedly reduced cardiac events. Survival is identical to the expected survival, including the post-operative follow-up period. Thus, surgery has restored life expectancy.
Conclusions
Risk stratification using quantitation of MR and ASE classification is a powerful predictor of outcome, supersedes all qualitative criteria, and defines a high –risk group (patients with an ERO >40 mm2). In patients with an ERO >40 mm2), under medical management the mortality and morbidity is considerably increased and there is excess mortality. Surgery normalizes life expectancy. In patients with AMR, LV dysfunction and left atrial enlargement are weak predictors, and early surgery should be offered to patients based on an ERO >40 mm2.
Timing for Operation for Asymptomatic Severe Aortic StenosisA Jamil TajikMayo Clinic
|
The concerns about asymptomatic severe aortic stenosis include left ventricular (LV) hypertrophy resulting in subendocardial ischemia, LV diastolic dysfunction, LV systolic dysfunction, ventricular dysrhythmias, and sudden death.
Risks and benefits of surgery in asymptomatic AS
Tajik, Pellikka and colleagues studied 622 patients (238 women, 72 years old) with AV velocity >4 m/s, a mean gradient of 46, and an AVA of 0.9 cm2. Sudden death occurred in 11 (4%) of the 270 patients with asymptomatic, hemodynamically significant AS and did not have surgery—a higher rate than the operative mortality in this population. Survival of patients who were censored at surgery was significantly inferior than the survival in the age- and sex-matched population (30% vs about 50%; p<0.001).
|
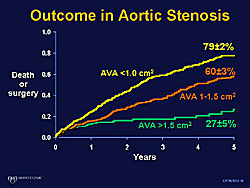
Three important studies in recent years addresses asymptomatic severe AS. All patients had a velocity > 4 m/sec (Figure 1). An 80% event rate (death, aortic valve replacement) in the first 2 years of follow-up was found in the 123 patients (63 years old) reported by Otto and colleagues. Rosenhek and colleagues found a 44% event rate in 128 patients (60 years old), and Amato and colleagues found a high 6% incidence of sudden death in 66 patients (50 years old).
In the study by Rosenhek and colleagues the event-free survival was 67% at 1 year, 56% at 2 years, and 33% at 4 years in symptomatic patients. Further, they found that the rate of progression of aortic jet velocity was strong predictor of future events. Also, the event rate was higher in the patients with more valve calcification. Event-free survival was 75% with no or mild calcification, and 20% with moderate of severe calcification.
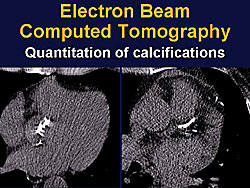
Now there is a very precise measurement by CT scanning to detect and measure the volume of calcification of the aortic valve. A study by Sarano, Tajik and colleagues showed that the amount of the aortic valve relates very well to the severity of the AS, and is an independent predictor of outcome (Figure 2). Hence, the severity of calcification is now used at Mayo Clinic in weighing the decision to perform surgery in this population.
Amato and colleagues studied the role of exercise testing in the treatment decision for patients with asymptomatic AS. They studied 66 patients (50 years old, 68% male), and an AVA ?1.0 cm2 (mean 0.61, range 0.30-1.0 cm2). Exclusions from the study included dysrhythmias or left bundle branch block, presence of coronary artery disease (CAD) on angiography, other valvular disease, and comorbid diseases. Sudden death occurred in 4 of the 66 patients before 1 years, despite being followed-up every 3 months and being instructed to report any symptoms.
Surgical risk
In the Society of Thoracic Surgeons National Database, the operative mortality was 3.61% of 9095 patients who underwent total valve replacement surgery (8767 survived surgery, 328 operative mortalities). In the 880 patients with NYHA class I, 869 (88.75%) patients survived surgery and 11 (1.2%) patients died; this operative mortality was lower than the risk of sudden death.
The indications for early aortic valve replacement surgery in asymptomatic severe AS are:
- Progressive increase in AV velocity and decrease in AVA
- LV systolic dysfunction
- Marked LV hypertrophy
- Associated severe CAD
- Ventricular dysrhythmias
- Significant diastolic dysfunction
- Noncardiac surgery
- Strong patient desire for physical activity
- Patient preference (many older patients would prefer surgery earlier than later)
Mitral Valve Repair for Patients with Severe Mitral Regurgitation and Sinus RhythmYukikatsu OkadaKobe General Hospital
|
Echocardiography reveals the mechanism of regurgitation and the size and area of the regurgitant orifice. Surgical procedures are becoming reproducible and durable. For the last decade, with intra-operative transesophogeal echocardiography (TEE) it is possible to immediately assess surgical results and quality of repair in the operating room.
Most degenerative mitral regurgitation (MR) has been repairable over the last 10 years. Surgical indications include signs of heart failure (early NYHA class II), left ventricular LV) systolic dimension up to 40-45 mm, and cardiac rhythm just before and after atrial fibrillation.
Outcomes after repair of degenerative MR
|
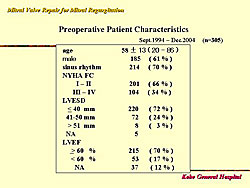
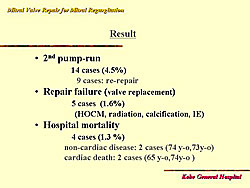
From 1994 to 2004, at Kobe General Hospital, mitral valve repair was possible in 89% of all cases, and in 98.4% of patients with myxomatous degeneration. Figure 1 shows the baseline characteristics of these patients. The types of procedures used in these patients are shown in Figure 2. Concomitant procedures was required in 112 (38%) of patients: Tricuspid annuloplasty in 51, MAZE in 28, aortic valve replacement in 14, and coronary artery bypass surgery in 11, and other in 10 patients. The outcomes in these patients are shown in Figure 3. Death occurred in 4 patients (1.3%), of which 2 were non-cardiac deaths. The mean follow-up was 52 months.
|
|
|
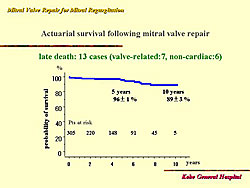
At 10 years, the probability of survival was 89% (Figure 4). Age > 70 years of age was shown to be a significant risk factor for survival on univariate analysis. However, on multivariate analysis, this was not significant. At 6 years, survival was 96% for patients
< 70 years of age, and 84% for patients ≥70 (p=0.0004).
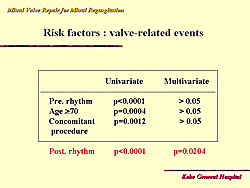
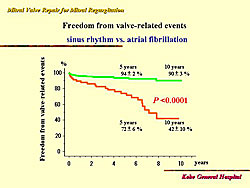
Further analysis showed no significant difference for survival between patients with atrial fibrillation and normal sinus rhythm (about 95% for both). Freedom from re-operation after mitral valve repair was 99% at 5 years and 10 years. But, freedom from valve-related events at 5 years after repair was 88% and 75% at 10 years. The risk factors for valve-related events are shown in Figure 5. Only sinus rhythm and atrial fibrillation were significant predictors of valve-related events, with 10-year freedom from valve-related events 42% with atrial fibrillation and 90% with sinus rhythm (Figure 6).
|
|
Is atrial fibrillation predictable?
|
||
|
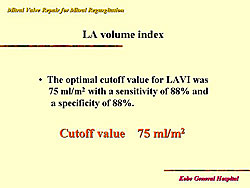
Okada and colleagues showed that left atrial volume index was the most significant parameter to predict atrial fibrillation. They analyzed left atrial size, left atrial dimension, dimension index, left atrial volume, and volume index. Then, they analyzed 66 patients at the diagnosis of severe MR, and found that over the follow-up period 8 patients developed atrial fibrillation. They concluded that the optimal cut-off value for left atrial volume index was 75 ml/m2 (Figure 7).
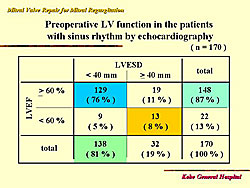
LV function in patients with sinus rhythm and severe MR
In 170 cases from 1994 to 2004, most had a 60% ejection fraction and a 40 mm LV end systolic diameter. The pre-operative LV function is shown in Figure 8.
Summary
Severe degenerative mitral regurgitation is repairable, even in patients with prolapse of both valves. Operative morbidity and mortality is low, even in patients less than 70 years old. Long-term outcomes (valve-related events, reoperation) are excellent in patients with sinus rhythm. Surgical outcomes are best in patients who are mildly symptomatic. Echocardiographic measurements and cardiac rhythm are useful to guide the timing of surgery in asymptomatic patients.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |