|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Atrial Fibrillation: Therapeutic InnovationsMaria Belen O. CarismaPhilippine Heart Center
|
Atrial fibrillation (AF) is a complex arrhythmia that can resolve on its own, recur, or become persistent. AF is a disease of the elderly, with about 70% occurring in persons aged 65 to 85 years old. The Framingham Heart Study estimated that the lifetime risk for AF after 40 years of age is 1 out of 4.
Pharmacologic treatment of AF
|
Available anti-arrhythmic drugs, based on the von Williams classification, are shown in Figure 1. Classes II and IV are primarily used for rate control and classes I and III for rhythm control. The choice of drug is based on the degree of structural heart disease and the underlying risk of pro-arrhythmia.
Rate control versus rhythm control as the better approach has been a controversial subject in recent years. Four clinical trials, shown in Figure 2, all seem to conclude that rhythm control offers no benefit over rate control. However, caution should be used before generalizing this conclusion to all patient populations. Some patient subsets will benefit more from rhythm control:
- Patients < 65 years of age with lone AF
- New onset AF
- Persistent AF who are highly symptomatic, or poor left ventricular (LV) function.
New anti-arrhythmic drugs that are in late phase clinical trials are dronedarone, trecitilide, tedisamil, and azimilide (Figure 3).
|
|
Non-pharmacologic treatment of AF
Two current notions of the mechanisms of AF serve as the basis of its non-pharmacologic treatment: the multiple wavelet hypothesis, and the focal mechanism with fibrillatory conduction concept.
The multiple wavelet hypothesis states that AF occurs if there is sufficient mass of atrial tissue resulting in a self-perpetuating activity. Cox-Maze surgery developed on the basis of this hypothesis. The focal mechanism theory states that if a single focus is firing very rapidly the rest of the atrium cannot conduct in a 1:1 fashion and this will result in chaotic atrial activation.
Haissaguerre observed in the mid 1990s that there are focal triggers within the pulmonary veins (PV) upon which catheter ablation could be applied to cure AF. The autonomic nervous system also contributes to AF, particularly the parasympathetic system.
Non-pharmacologic approaches include:
- Surgical Maze
- Catheter Ablation
- Pacing
- Implantable Atrial Defibrillators
- AV Nodal Ablation and Pacing
Surgical maze was developed in 1987 and created in both atria ablation lesions using the scalpel. The undesirable clinical sequelae of Maze I led to refinements found in Maze II and II. Maze III achieved a high clinical efficacy of nearly 90% in centers that performed it regularly, however, there were drawbacks to this procedure. In particular, it required a certain degree of skill and not all cardiac surgeons could perform the procedure, the procedure was extensive and required significant cardio-pulmonary bypass time resulting in significant morbidity. Also, the risk of morbidity was difficult to justify, given that AF is not a life-threatening disease.
Thus, novel ablation energy technologies were developed:
- Unipolar and bipolar radiofrequency systems
- Microwave energy
- Ultrasound energy
- Lasers
- Cryothermy.
Percutaneous Maze procedure replaced surgical Maze, and the scalpel was replaced by RF ablation was used to create the ablation lesions in both atria. The success rates varied by centers, ranging from 22-50%. This approach was also technically demanding, time consuming, with high fluoroscopy exposure, and frequent gaps that resulted in pro-arrhythmic events. Also, there was an unacceptably high rate of complications, including thromboembolism. Eventually, this approach was abandoned.
The focus of ablation then turned to PVs based on Haissaguerre’s study and the finding that 95% of foci of AF were in the PVs. This called for introduction of catheter into the atria and into multiple pulmonary veins and targeting spontaneous atrial discharges or PV muscle potentials and delivering catheter ablation. This approach initially showed promise, but later studies showed limitations. First, it required firing of the triggers, which could be hours, and thus resulted in a long duration of procedure time. Second, a high dose of isoproterenol that led to multiple AF inductions and multiple cardioversions. Third, recurrences were common, and fourth, there was high risk of developing PV stenosis.
The current goals are to empirically all of the PV to improve efficacy and to isolate the PV as proximal to the left atrial tissue as possible to minimize the risk of PV stenosis. Two broad strategies have been adopted based on these goals. One is segmental PV isolation, in which RF energy is delivered in 2 segments of the PV ostium. The second approach is a circumferential approach.
Imaging is needed to provide an anatomical roadmap. Computed tomography (CT) and magnetic resonance imaging (MRI) are used before the procedure, after which the data are segmented to extract to the PV anatomy, followed by registration of the 3-dimenstional data set into an electro-anatomical mapping system, which are done during the procedure so the operator may have real-time visualization and manipulation of the catheters.
The technical difficulty of performing point-to-point linear lesions has led to the development of balloon-ablation devices to rapidly isolate the PV. These include a cryoablation balloon catheter, laser ablation catheter, focused ultrasound balloon ablation catheter, and are in clinical trials in Europe.
No large randomized clinical data is available catheter ablation to compare the efficacy of anti-arrhythmic drugs versus catheter ablation. Success rates of catheter ablation range from 60-90%.
Pappone and colleagues studied 1171 patients on pure anti-arrhythmic drugs who underwent catheter ablation. The endpoints were mortality, morbidity, and recurrent AF. This single-center, non-randomized study showed that AF recurrence was significantly lower in patients who had ablation compared to medical therapy (20% vs 50%, respectively). Mortality was lower because of a lower rate of cardiovascular deaths, congestive heart failure, stroke, and sudden death.
The CACAF (Catheter Ablation for the Cure of Atrial Fibrillation) study reported at the American College of Cardiology meeting in March 2005 showed the superiority of catheter ablation over medical therapy; only 44% of the ablation patients had at least 1 atrial tachyarrhythmia, compared to 91% in the medical therapy group (p<0.001), over the follow-up. The risk of recurrent atrial arrhythmia was 3.2-times higher in the medical therapy group over the average 12.5 months follow-up. This is first prospective, randomized controlled trial to compare these two treatment approaches.
Regarding pacing, the American Heart Association Council on Clinical Cardiology Advisory issued an advisory that stated, “Permanent pacing to prevent AF per se, is not indicated, unless patients have a concomitant bradycardia indication.”
Implantable atrial defibrillators have not been used much, primarily because of the discomfort of the shocks. The use is limited to patients who are highly symptomatic and drug refractory.
AV node ablation followed by pacing is another therapeutic option. Traditionally, right ventricular pacing follows targeted destruction of the AV node. While right ventricular (RV) pacing alters the natural sequence of ventricular activation and contraction, bi-ventricular (BV) pacing corrects this mechanical dys-synchrony, especially in patients with congestive heart failure and conduction system disease.
The PAVE study, reported at the 2004 American College of Cardiology meeting compared RV and BV pacing in patients undergoing AV node ablation regardless of LV systolic function or NYHA class. Exercise capacity was the primary endpoint. In this first prospective, randomized study of these two approaches, there were more deaths in the RV group than the BV group. Only the BV group had sustained improvement at 6 months in the primary endpoint, although both groups had improvement in walking distance. The authors concluded that in patients with chronic AF treated with AV nodal ablation, BV pacing produces a statistically significant improvement in functional capacity, when compared with RV pacing, as measured by the 6-minute walking test, peak VO2, and exercise duration. This improvement reflects a sustained benefit in the BV group rather than a deterioration in the RV pacing group. The results suggest that BV pacing should be the preferred mode of therapy in patients undergoing AV nodal ablation for the control of chronic AF.
ARB as an Upstream Therapy for Preventing Atrial FibrillationKoichiro KumagaiFukuoka University
|
|
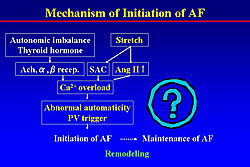
Jais reported that left ventricular end-diastolic pressure (LVEDP) was significantly higher in patients with lone atrial fibrillation (AF) than that in the control. The LV diastolic dysfunction induces the left atrial (LA) stretch and enlargement, then resulting in pulmonary vein (PV) stretch and PV foci. In the cardiomyocyte, stretch activates stretch activated channel (SAC), and also activates acetylcholine, alpha or beta stimulation, and angiotensin II, binding each receptor and inducing calcium overload. Angiotensin II activates the MAP kinases and thereby promoting fibrosis. The mechanism of initiation of AF is shown in Figure 1. However, this does not explain the maintenance of AF, which may be explained by remodeling.
EP studies of chronic AF
An electrophysiology (EP) study after cardioversion of chronic AF (CAF) by Kumagai and colleagues showed that with CAF had a shorter ERP and slower conduction velocity (CV) than the control, which reentry to form easily.
In simultaneous multisite mapping during induced AF in the sterile pericarditis model by this group showed that unstable reentry circuits that disappeared and reformed were frequently observed. The greater the number of unstable reentry circuits the longer the duration of AF.
Recently, they analyzed the EP properties in the PV in humans using basket catheter mapping. Programmed pacing was performed from the distal electrode pair. In one case shown by Kumagai, the ERP of the distal PV was 60 ms. When S1-S2 was decremented to 80 ms, the conduction time from the distal to proximal PV was 136 ms. Thus, PV has a very short ERP and conduction delay.
Analysis of the activation map of the PV using the basket catheter showed that a wave front from a focal discharge in the PV goes through the left atrium (LA) nearest exit breakthrough point, and turns around at the PV-LA junction, and re-enters through the PV from the entrance site, forming a reentrant circuit. This group first demonstrated the PV-LA junctional reentrant circuits in humans.
Kumagai thinks the PVs act not as focal triggers, and also as a driver, for example, of PV-LA junctional reentry or reentry in the PV. This may act as a substrate for AF maintenance.
AF begets AF
The concept of AF begetting AF was devised by Wijffels. The AF duration is progressively prolonged because the ERP is shortened. The ERP is progressively shortened because the intracellular calcium overload during high-frequency atrial activation decreases the calcium current, and thereby shortens the APD.
Intracellular calcium is regulated through the intake of extracellular calcium and released from sarcoplasmic reticulum in myocytes via the activation of membrane L-type calcium channel or phospholipase C pathways. Angiotensin II activates both the L-type calcium channel and the intake and release of Ca. Thus, intracellular Ca overload induced by angiotensin II might play a role in electrical remodeling. Therefore, this group hypothesized that an ACE inhibitor or angiotensin receptor blocker (ARB) could attenuate calcium overload and prevent electrical remodeling.
Studies of ARB and electrical remodeling
Kumagai and colleagues studied the effect of the ARB candesartan on short-term electrical remodeling, and showed that candesartan prevented the electrical remodeling induced by short-term rapid atrial pacing. This is the first report of the relationship between renin-angiotensin system and AF. However, the effects of candesartan on long-term electrophysiological and structural remodeling remain unclear.
Wijffels et al evaluated AERP shortening and inducibility of AF over 2 weeks. Their data showed that AERP was shortened within the first 24 hours. In contrast, AF was not induced until after 1 week. So, there is a discrepancy in the time course of these changes. Thus, the maintenance of AF cannot be explained by only ERP shortening.
To determine the other mechanisms contributing to the maintenance of AF, Kumagai and colleagues evaluated the long-term effect of rapid atrial pacing at 400 bpm for 5 weeks in a canine model. The EP study was performed every week to measure ERP, conduction time, and AF duration. At the end of the experiments, the pathological study was performed.
In both the control and candesartan groups, AERP shortening was most pronounced after 1 week, and continued during pacing. The degree of AERP shortening after rapid pacing was not significantly different between the two groups. However, the AF duration in the candesartan group was significantly shorter than that in the control group.
In the control group, the intra-atrial conduction (CT) was gradually prolonged, but this did not occur in the candesartan group. The percentage of fibrosis in all atrial regions in the candesartan group was markedly lower than that in the control.
Representative histological sections of the right atrial free wall from each group showed that in the control group, after 5 weeks of pacing, there was extensive interstitial fibrosis in the controls, but it was attenuated in the candesartan-treated animals. In normal atria, conduction is fast, but in the atria with fibrosis, conduction is delayed, forming reentrant circuits, maintaining AF. However, candesartan can prevent the fibrosis and thereby conduction delay and maintenance of AF.
Some clinical evidence to support this comes from the Val-HeFT and CHARM studies. Valsartan and candesartan significantly decreased the new onset of AF in CHF compared with placebo in these studies. In the LIFE study, losartan significantly decreased by 28% the incidence of AF in hypertension compared with atenolol.
Madrid and colleagues studied the effect of irbesartan on maintaining sinus rhythm after conversion from persistent AF. The patients treated with amiodarone plus irbesartan had a significantly lower rate of AF recurrence, compared to treatment with amiodarone alone.
In another study, endomyocardial biopsies of LV were performed before and after 1 year of treatment with losartan in patients with hypertension. Losartan significantly regressed myocardial fibrosis and may have a reverse remodeling effect. This study may indicate that an ARB has a reverse remodeling effect.
Summary
|
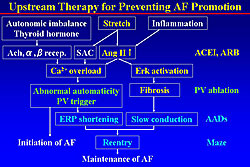
Kumagai and colleagues hypothesize that the mechanism of maintenance of AF involves stretch and inflammation increasing the levels of angiotensin II, which increases the level of calcium and promoting abnormal automaticity from the PV, thereby initiating AF (Figure 2). Angiotensin II activates the Erk cascade, promoting fibrosis and inducing slow conduction. ERP shortening plus slow conduction allow easy formation of reentry circuits and maintain AF. ACE inhibitors or ARBs can be targeted at stretch, angiotensin II, calcium overload, Erk formation, and fibrosis. The PV was treated by PV ablation, and anti-arrhythmic drugs prolong the ERP and blocks the conduction to prevent the reentry formation. Thus, ARBs may be an upstream therapy for preventing AF promotion.
Mechanisms of Atrial Fibrillation: Role of the Posterior Left Atrium and Pulmonary VeinsGraeme SlomanThe Royal Melbourne Hospital & Epworth Medical Centre
|
The three mechanisms of atrial fibrillation (AF) are focal origin, single reentry, and multiple reentry. AF can be either focal or chronic. The consequences of AF can be either electrical and become sustained AF, or structural with dilatation and damage of the atrial muscle or complete remodeling of the myocardial chambers.
Early work by Haissaguerre identified the location of the focal origin of the atrial ectopic beats. The majority arise from the pulmonary vein (PV), although some may arise from the right atrium, superior vena cave, inferior vena cave, which are irritable foci that can be the precursors of the establishment of AF. In a study of 45 patients by Haissaguerre, 62% were fee of AF at 8 months after ablation of the foci in the PV.
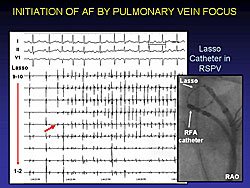
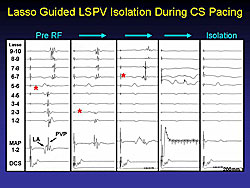
The irritable foci that can produce rapid AF can be identified by taking multiple recordings through a Lasso catheter in the PV, as shown in Figure 1 and Figure 2. This is the basis of much of AF ablation therapy.
|
|
|
With the multiple electrode catheters, it is possible to obtain rapid atrial or PV tachycardia at very rapid rates with the Lasso in the PV (Figure 3). Yet, on a surface electrocardiogram the patient may appear to be in sinus rhythm. Patients without AF have not been studied to determine have the same pattern. It may well be that quite a few people with the occasional atrial ectopic beat may have fast tachycardia in the PV, which does not become evident in the person’s clinical condition or on routine electrocardiograms.
The majority of the irritable foci occur in the PV or in the area between the PV and the left atrium (LA). In a study by Lin and Sloman, in 240 patients they found that 72% of the patients had foci in the PV-LA junction, and 28 had foci that occurred outside the PV.
Special imaging techniques can be used to visualize the direction of the conduction from the atrium. Also, these images have shown that in the left atrial and the proximal portions of the PV there is a lot of muscle tissue, which is relatively thick. It is thought that irritable foci, that initiate AF, reside in this muscle tissue. The anatomy of the PV shows there are muscle “islands”. Also, in the layers of the lining of the wall of the PV, there are layers of myocardium, which can be the focus for irritable beats. High-resolution optical mapping can assess the arrhythmogenic substrate of the PV muscle wall.
The PV-LA junction has been thoroughly investigated. Kumagai and colleagues have shown there is ERP heterogeneity and anisotropic conduction at the PV-LA junction. In 48 patients with paroxysmal AF, using a basket catheter, they were able to show the direction of the stimulation from the PV.
|
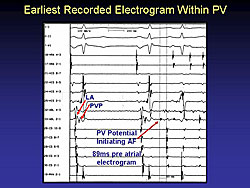
Pulmonary vein electrophysiology (EP) has provided insights into the focal activity of paroxysmal and sustained AF (Figure 4). The mechanisms for focal can be either because of abnormal automaticity, triggered activity, or reentry through in the LA junction. The reentrant mechanism led to the development of the Maze procedure, either surgical or ablation, which can prevent recurrence.
Conclusions
Focal activity within the PVs are the usual triggers for AF (non-PV sites less frequently). Atrial muscle sleeves extending onto the veins appear to harbor these triggers for AF. Reentry or single microreentrant sources anchored at the PV-LA junction may serve as ongoing drivers for AF. The complex anatomy and the heterogeneous EP properties of the PV-LA junction appear to promote reentry. Advanced atrial structural disease may facilitate multiple wavelet reentry; however, still probably a dependence on the posterior LA.
Cure of Atrial Fibrillation by Extensive Encircling Isolation of Ipsilateral Pulmonary Veins (EEPVI) Associated with Aggressive Search & Ablation of Extra-Foci.Yoshito IesakaTsuchiura Kyodo Hospital
|
Catheter ablation technique to cure atrial fibrillation (AF) based on isolation of the pulmonary vein (PV) still remains under development, because of its potential risks of severe PV stenosis and obstruction, atrio-esophageal fistula and the genesis of reentry atrial tachycardia, and its limited long-term success rate. These are possibly related to the complex anatomy and electrophysiology of the PV and its junction to the left atrium (LA) and also the existence of non-PV foci. Iesaka and colleagues developed a new technique of electroanatomy-guided extensive encircling isolation of ipsilateral PVs (EEPVI) in conjunction with aggressive search and ablation of non-PV foci.
|
||
|
||
|
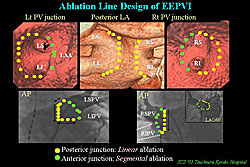
In their EEPVI procedure, the ablation line design is illustrated on 3D-CT and selective angiography of PV (Figure 1). At posterior LA-PV junction, linear ablation is performed at 1 to 2 cm outside PV, while at anterior junction, segmental ablation is performed on the ridge dividing the anterior PV wall and surrounding tissues.
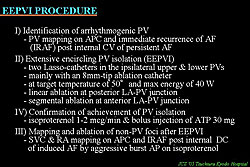
The steps to the EEPVI procedure are listed in Figure 2. First, arrhythmogenic PVs are identified by PV mapping on IRAF after internal cardioversion. Then, EEPVI is performed according to the protocols listed in the figure. Achievement of PVI is confirmed by bolus injection of ATP 30 mg during isoproterenol infusion. Finally, SVC and RA mapping is done post internal DC of induced AF.
In two cases shown by Iesaka, double Lasso catheter monitoring of ipsilateral PVs during EEPVI demonstrated simultaneous disconnection of upper and lower PVs. This was seen in more than 50% of the cases.
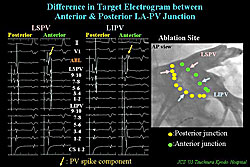
For EEPVI, the target sites are determined based on multi-plane PV angiograms and local electrograms. At the posterior LA-PV junction, the target electrogram is a large LA potential, while, at the anterior junction, target electrogram is complex potential consisting of LA component and spiky PV component superimposed (Figure 3).
Accordingly, there are different ablation endpoints concerning local electrograms between anterior and posterior junctions. At the posterior junction, the endpoint is the marked reduction of the electrogram-amplitude and formation of narrow-double potential. At the posterior junction, the endpoint is the diminished or marked delay of PV component associated with marked delay of intra-PV conduction.
The EEPVI technique has the potential for modifying the substrate, because of the ablation at the LA-PV junction.
During ablation at the posterior LA-PV junction in EEPVI, persistent AF is terminated in about 40% of the patients. Notably, prior to termination of AF, stabilization and slowing of intra-PV and intra-CS activation occurred. In the remaining 60% of patients, after cardioversion AF re-initiation was completely suppressed despite the persistence of frequent PV firing
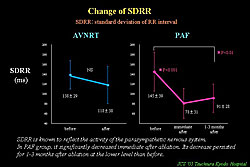
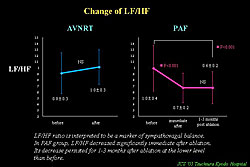
EEPVI also has the potential for autonomic nerve tone modification, represented by withdrawal of vagal nerve tone. Marked and abrupt HR increases commonly occur during ablation at anterior junction of RSPV, and this increase persists after procedure. Heart rate variability parameter, SDRR, which reflects parasympathetic nerve tone, is significantly decreased and persisted for several months after the procedure (Figure 4). LF/HF ratio, a marker of sympathovagal balance, significantly decreased and persisted for more than 1 month (Figure 5). This withdrawal of the vagal nerve tone is thought to be because of thermal damage of the fat pad existing RSPV and SVC during ablation.
|
|
To try to reduce the reconnection of isolated PVs, they provoke dormant PV conduction after PV disconnection by ATP bolus injection during the infusion of high dose of isoproterenol. In one case shown by Iesaka, transient reconnection of PV was seen at the phase of maximum effect of ATP. After additional RF energy, the reconnection of PV became non-inducible by the same provocation. This procedure will help to reduce the recurrence of PV induction.
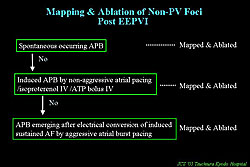
After EEPVI is completed, they tried to induce AF by aggressive atrial burst pacing, to search for and ablate non-PV foci after cardioversion of sustained AF, using the protocol shown in Figure 6. For SVC trigger, SVC disconnection was principally performed. Non-PV foci were identified in 17% of patients. SVC proved to be the most dominant site of non-PV foci. The mean success-rate of ablation of these foci was 73%, which is relatively low.
Results of EEPVI and non-PV focal ablation are shown in Figure 7. All the ATs post EEPVI was due to PV ostial reentry caused by gap in ablation line, and those were easily ablated by focal ablation.
|
|
Summary
Extensive encircling PV isolation in conjunction with aggressive search and ablation of non-PV foci ablation was relatively safe and highly effective, possibly due to the favorable electro-physiologic modification of LA-PV junction as substrates for AF initiation and perpetuation, in addition to elimination of AF triggers. EEPVI was associated with possibly favorable autonomic tone modification.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |