|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Noggin Promotes Cardiomyocyte Differentiation in Murine ES CellsKeiichi FukudaKeio University School of Medicine
|
The clinical application of embryonic stem (ES) cell technology has been impeded by a lack of reliable and selective differentiation methods for inducing cardiomyocyte generation. Dr. Keiichi Fukuda, Keio University School of Medicine, presented the results of a study to develop a protocol for inducing differentiation of cardiomyocytes from ES cells using internal regulatory signals.
Whole mount in situ hybridization in the heart-forming region at the cardiac crescent area was done to detect factors involved in cardiomyocyte differentiation. The bone morphogenic protein (BMP) inhibitor, Noggin was transiently and strongly expressed in the cardiac crescent from embryonic day 7.5 (E7.5) to E8.25. Noggin also was expressed at the notochord after E8.5.
|
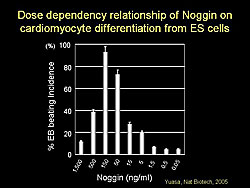
Using this information, a protocol was developed to induce cardiomyocyte differentiation using Noggin. Two ES cell lines were cultured and Noggin was added at day -3, day 0, and various time points after embryoid body (EB) formation. Administration of Noggin at day -3 and day 0 strongly augmented the incidence of beating EBs. The effect of Noggin on cardiomyocyte differentiation was dose-dependent, with 150 ng/mL providing the highest incidence of beating EBs, while higher concentrations inhibited differentiation (Figure 1).
The addition of BMP2 to the culture inhibited cardiomyocyte induction in a dose-dependent manner. Addition of other BMP inhibitors, including chordin and BMPR1A also induced cardiomyocyte differentiation in ES cells. These findings indicate that inhibition by Noggin at an early time point is important for cardiomyocyte induction.
Immunofluorescent staining of EBs showed that Noggin treated EBs strongly expressed myosin heavy chain (HC), atrial natriuretic peptide (ANP), troponin 1, and actinin in contrast to control EBs, which showed only slight expression of these genes. Transient treatment with Noggin markedly enhanced the expression of cardiac transcription factors and cardiac specific proteins.
|
||
|
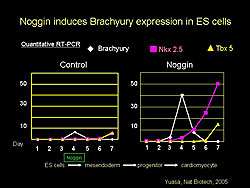
Noggin induced Brachyury expression in ES cells and not in control cells. Following Brachyury expression, Noggin-treated cells also expressed Nkx25 and Tbx5 (Figure 2). Noggin appears to work at the point when ES cells differentiate into mesoendoderm.
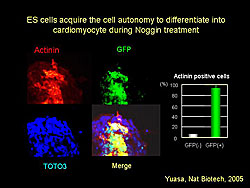
Immunostaining of ES cells cultured with Noggin with and without GFP showed that most GFP(+) ES cells expressed actin, while GFP(-) ES cells did not (Figure 3). This finding demonstrates that ES cell differentiation into cardiomyocytes is a cell autonomous process.
Dr. Fukuda concluded that Noggin is transiently expressed in the heart-forming area from E7.5 to E8.25. Transient and strong inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation in ES cells in a cell autonomous manner.
Myocardial regeneration: Basic Biology and Therapeutic ProspectsGordon TomaselliJohns Hopkins University School of Medicine
|
Dr. Gordon Tomaselli, Johns Hopkins University School of Medicine, reviewed clinical imperatives and therapeutic goals for developing stem cell (SC) therapy for myocardial disease. Potential cell sources for cardiac repair include bone marrow (BM) progenitor cells, endothelial precursor cells, embryonic stem cells (ESCs), and cardiac stem cells (CSCs). Cardiac regeneration from SCs requires mobilization, homing or direct delivery, and adhesion and migration of the SCs, resulting in tissue neogenesis.
Studies have shown that murine ESCs transdifferentiate and engraft in mouse models and human ESCs (hESCs) transdifferentiate and engraft in the pig heart. ESCs have plasticity, longevity, and capacity for renewal; however, limitations include ethical concerns, possibility of teratoma formation, chromosome abnormalities, exposure to animal products in culture, and immunogenicity.
BM derived cells include hematopoietic stem cells (HSC), side-population (SP) cells, and mesenchymal stem cells (MSCs). MSCs are multipotent, they grow and expand readily, and are genetically stable. Labeled MSCs injected into normal and infarcted animal hearts expressed cardiac specific markers but differentiation was incomplete. Despite this, MSC implantation has produced significant functional benefit in various infarct models. In a pig model, numerous labeled MSCs were detected in hearts of the treated pigs compared to none in the control hearts. LV function improved in the treated pigs eight weeks after infarction.
In 2003, researchers found clusters of CSCs in myocardium, showing that the heart is a self-renewing organ. Cultured cells from human myocardium produce clonogenic, oligopotent 3-D cardiospheres that proliferate, contract, and express cells of all types from the cardiac lineage.
Choice of SC type for therapy in humans depends on the biology of the stem cells and on the specific clinical settings and therapeutic goals. Since 2000, numerous clinical trials of SC therapy in acute myocardial infarction (AMI) and ischemic cardiomyopathy have been conducted, mostly with BM cells. Results have been mixed, depending on the cell lines and methods used.
A trial of MSCs in patients with AMI is nearing completion at Johns Hopkins University. This multicenter, double-blind, randomized trial is investigating safety, dosing, and MRI indices of function in patients receiving allogeneic MSCs by IV delivery. A total of 48 patients have been enrolled so far and no serious adverse events have been reported.
Dr. Tomaselli concluded that SCs offer great promise for cardiomyopathy treatment. The available cell types have complimentary attributes and liabilities. However, the mechanisms by which cardiac function is improved are still debated. Protocols for isolation, processing, and delivery of SCs should be standardized. The role of interventions to enhance SC function and homing requires further study.
|
|
|
Copyright © 2006 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |