|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Mechanism and Pathophysiology of Ischemic Mitral Valve RegurgitationYutaka OtsujiKagoshima University
|
Ischemic mitral regurgitation (MR) develops in about 20% of patients with acute myocardial infarction (AMI). Even mild ischemic MR worsens patient survival. Dr. Yutaka Otsuji, Kagoshima University, discussed the physiological changes that lead to ischemic MR and the need for treatment interventions targeted to these mechanisms.
Otsuji studied the relationship of leaflet tethering, papillary muscle (PM) function, and MR in patients with inferior MI. A patient with significant MR and good PM function had systolic thickening. Tissue Doppler strain imaging showed good longitudinal shortening. The distance between the PM tip and anterior annulus was long and the leaflet tented. In contrast, a patient with minimal MR and poor PM function had a thin, long PM and no systolic thickening. Tissue Doppler showed dyskinetic PM motion. In this case the PM tip to the annulus distance was relatively short with only mild leaflet tenting and MR. These comparisons demonstrate that PM dysfunction attenuates leaflet tethering.
Otsuji evaluated the role of annular dilation in MR. A patient with a small annulus from ring annuloplasty had an enlarged ventricle with leaflet tenting and significant ischemic MR. A second patient with lone atrial fibrillation had a dilated annulus but good LV function and almost no MR. Thus, annular dilatation alone does not cause significant MR. Frequent discrepancies between annular dilatation and MR suggest annular dilatation may be a weak factor.
|
||
|
||
|
In patients with ischemic MR, anterior mitral leaflet (AML) and posterior mitral leaflet (PML) tethering increases symmetrically. Patients with recurrent MR after annuloplasty have asymmetrically increased AML and PML tethering, with most of the coaptation on the posterior side. As in the case shown, anteriorly directed jet is occasionally seen (Figure 1). Surgical annuloplasty reduces the anterior-posterior annulus diameter. It does not displace the anterior annulus, but does displace the posterior annulus anteriorly, causing no change in the anterior leaflet and augmented tethering of the posterior leaflet (Figure 2). The increased posterior leaflet tethering may be related to recurrent ischemic MR after surgical annuloplasty.
A case study of surgery to reduce tethering was presented by Otsuji. A patient with dilated cardiomyopathy and end-stage CHF presented with a tethered mitral leaflet with completely restricted motion. Mitral annuloplasty and surgery to realign the PM were performed. Post-operative echocardiography demonstrated marked reduction in tethering with significantly improved mitral leaflet motion, elimination of MR, and clinical improvement from NYHA IV to II.
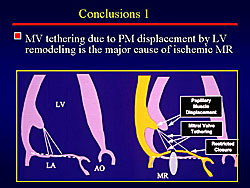
In conclusion, mitral valve tethering due to PM displacement by LV remodeling is the major cause of ischemic MR (Figure 3). Procedures that reduce tethering attenuate ischemic MR with clinical improvement.
Quantitative Assessment of Tethering by 3D EchocardiographyNozomi WatanabeKawasaki Medical School
|
Ischemic mitral regurgitation (MR) is commonly treated with annuloplasty, even though MR often persists after surgery. New surgical procedures have been developed to correct abnormal changes in leaflet geometry. However, current assessment with 2D echocardiography may not adequately measure leaflet tethering. Dr. Nozomi Watanabe, Kawasaki Medical School, described a new method for quantitative assessment of tethering by 3D echocardiography.
The mitral valve has a unique morphology with a saddle-shaped, curved annulus and curved mitral leaflets. Because of this geometry, 2D echocardiography for measuring tenting length or area may miss the maximum tethering site. Ten years ago, Watanabe’s group created 3D images of the mitral annulus using extracted images from multiple planes but this procedure was too time consuming and could not be used for 3D quantitation. With the advent of transthoracic real-time 3d echocardiography, a three-dimensional volumetric image of the heart and its structures could be visualized. However, this method could not be used for 3D quantitation.
|
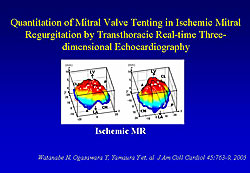
Watanabe and colleagues developed a software system for use with real-time 3D echocardiographic images, which provides quantifiable 3D images of the mitral leaflets and annulus. A real-time 3D echocardiographic study using this quantitative method showed that the mitral annulus flattens in ischemic MR. From these measurements, three-dimensional images of leaflets from normal mitral valves and mitral valves with ischemic MR were produced (Figure 1). The image from ischemic MR clearly shows the mitral valve tethering toward the left ventricle (LV) from the level of the annulus.
Watanabe’s quantitative method can be used to:
- measure the degree of tenting.
- tenting volume.
- tenting length.
- curve of the annulus.
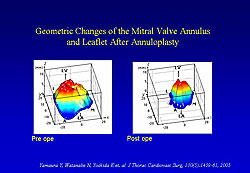
From these data, a quantitative map of mitral valve tenting can be made (Figure 2). Using this method to visualize and measure geometric differences in mitral valve tenting in different situations, they found that the tenting site is larger in patients with anterior infarction compared to those with inferior infarction. Images taken in a patient before and after annuloplasty showed that after surgery the annulus shrank in size and tenting volume was reduced but tenting height remained the same (Figure 3).
|
|
Watanabe concluded that the software can be expanded in the future to measure the entire mitral valve complex and analyze dynamics during the cardiac cycle. Using this technique, mechanisms of ischemic MR and individual variations can be evaluated. Importantly, surgeons can evaluate current surgical procedures and choose the best type of repair for individual patients.
| Footnote: Figures 1 and 2: Reprinted from JACC 2005;45:763-769. Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. Watanabe N, Ogasawara Y, Yamaura Y, Kawamoto T, Toyota E, Akasaka T, Yoshida K. With permission from American College of Cardiology Foundation. Figure 3: Reprinted from J Thorac Cardiovasc Surgery 2005;130(5): 1459-1461. Geometric change of mitral valve leaflets and annulus after reconstructive surgery for ischemic mitral regurgitation: real-time 3-dimensional echocardiographic study. Yamaura Y, Watanabe N, Ogasawara Y, Wada N, Kawamoto T, Toyota E, Akasaka T, Tanemoto K, Yoshida K. With permission from American Association for Thoracic Surgery. |
New Therapeutic Strategy for Ischemic Mitral Regurgitation: Repairing and Protecting LVMasashi KomedaKyoto University School of Medicine
|
Repair surgery for ischemic mitral regurgitation (IMR) is a challenge because it is a disease of the ventricle rather than of the valve. Dr. Masashi Komeda, Kyoto University School of Medicine, stressed that ventricular solutions are needed to effectively repair the defects that cause IMR. Surgical solutions used by Komeda include mitral annuloplasty (MAP) for annular dilatation; left ventricle restoration (LVR) for papillary dislocation; chordal cutting and translocation for leaflet tethering; and revascularization and resynchronization therapy when necessary.
MAP alone improves LV geometry and function. In an animal model of acute heart failure, MAP improved the LV short axis dimension (LVDd), wall motion in the direction of the short axis, and sphericity (LV became less spherical). Depending on the method used, LVR may distort LV shape. MAP with the SAVE procedure maintains the LV pear shape.
Strategies for IMR used at Kyoto University are complete revascularization, undersized MAP, LVR when indicated, and chordal cutting and translocation when necessary. In the past five years, 61 patients underwent procedures for IMR. Of these, 30 patients had MAP and coronary artery bypass graft (CABG) (MV repair group), and 31 had LV restoration, MAP, and CABG (LVR group). Mitral ring size used averaged 25 mm. LV repair methods were SAVE 12, Dor 20, and m-Batista 4.
|
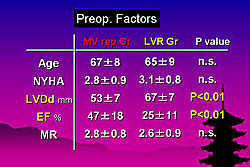
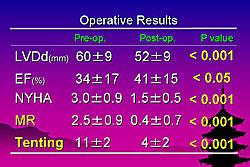
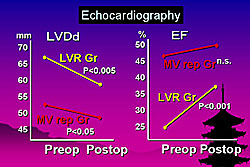
Preoperatively, patients in the LVR group had larger ventricle diameter (p <0.01) and lower ejection fraction (EF) (p <0.01) (Figure 1). Hospital mortality was 11% (7/61), with 6% (2/29) in the MV repair group and 16% (5/30) in the LVR group. After surgery, results of all patients showed that MR almost disappeared (p <0.001) and tenting significantly decreased (p <0.001) from baseline (Figure 2). The LVR group had greater improvement in LVDd and EF (Figure 3). Both groups had significant improvement in symptoms (p <0.001) and MR ((p <0.001).
|
|
A case study patient (46 year-old male) with a history of AMI developed MR with CHF. The ventricle was repaired with the Dor procedure, MAP was performed, and the secondary chordae were cut from the LV. Chordal translocation was done, connecting the papillary tips with the anterior mitral annulus using a new technique of chordal replacement. After surgery, the LV was much smaller with improved wall motion, leaflet tethering was decreased, and MR disappeared.
Komeda concluded that IMR should be treated as a ventricular disease. MAP and LVR can provide significant benefit for patients with poor LV function. Additionally, chordal reconstruction surgery may help improve the results
|
|
|
Copyright © 2006 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |