|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Relationship between Metabolic Syndrome and Early Stage Coronary Atherosclerosis (A 3-Dimensional Intravascular Ultrasound Study)Takashi HitsumotoToho University Sakura Hospital
|
Recent clinical studies using intravascular ultrasound (IVUS) have demonstrated that coronary artery plaque is already present in persons in whom this was not found on coronary angiography. Dr. Takashi Hitsumoto of Toho University Sakura Hospital and colleagues examined how the metabolic syndrome (MetSyn) influenced early stage coronary atherosclerosis using 3-D IVUS, particularly plaque vulnerability.
Figure 1 shows the study subjects and methods. A total of 70 subjects without stenotic lesion on coronary angiography (CA) were included, and key factors in the pathogenesis of the MetSyn were examined. Treatment for hyperlipidemia, hypertension, or diabetes was an exclusion criterion.
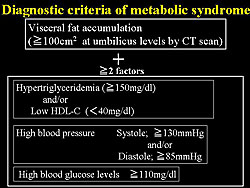
The Japanese criteria for the MetSyn were used in the present study (Figure 2). For the IVUS study, they observed 20 mm lengths of the proximal portion on the left anterior descending (LAD) coronary artery using auto-pullback methods of 1mm/sec velocity, and calculated the percent of plaque volume using the Netra-IVUS system as plaque quantity. Abnormal plaque quality such as eccentricity, calcification, and lipid pool into plaque was also evaluated. ?
|
|
|
||
|
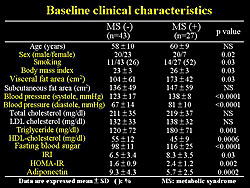
The baseline characteristics are shown in Figure 3. Nearly 30% were diagnosed with MetSyn, and their clinical data was mildly abnormal. HOMA-IR, a marker of insulin resistance, was significantly higher in the MetSyn subjects, while serum adiponectin concentration was significantly lower in subjects. Total cholesterol and LDL cholesterol levels were similar in both groups.
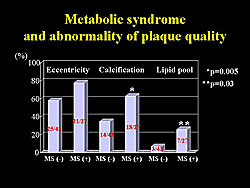
Percent plaque volume was significantly higher in MetSyn subjects (31±8 vs 21±8 without MetSyn; p<0.0001), demonstrating sufficient early stage plaque formation that is not detectable as stenosis on CA. A high incidence of abnormal plaque quality was also found in the MetSyn subjects, compared to non-MetSyn subjects (Figure 4).
A significant correlation was found between percent plaque volume and levels of trigylcerides, HDL-C, fasting blood glucose, and systolic and diastolic blood pressure. Also, a significant correlation was found with body mass index (BMI) and visceral fat area. No correlation was found between percent plaque volume and subcutaneous fat area, suggesting that visceral fat obesity is closely associated with plaque formation in the early stage.
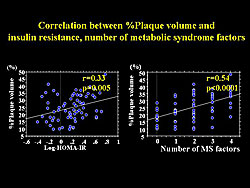
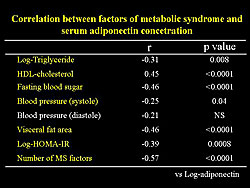
A significant relation between percent plaque volume and insulin resistance and the number of risk factors for the MetSyn was also found (Figure 5). A significant negative correlation between percent plaque volume and serum adiponectin concentration was found. However, serum adiponectin concentration had a significant correlation with each factor of the MetSyn (Figure 6).
|
|
Further analysis showed that a decrease in serum adiponectin concentration is closely associated with progression of early stage plaque formation, independent of MetSyn factors or presence of MetSyn. In MetSyn subjects, only serum adiponectin concentration had significant correlation with percent plaque volume. Thus, reduced serum adiponectin concentration is an important factor for plaque formation in persons with MetSyn at high risk population for cardiovascular events.
A high incidence of abnormal plaque quality, particularly calcification, was found in subjects with hypoadiponectinemia. Data from this group and others suggest that subjects with hypoadiponectinemia already have a vulnerable plaque not detected by CA.
In conclusion, Hitsumoto stated that their IVUS studied showed that subjects with the MetSyn, by Japanese criteria, represent a high-risk population, for vulnerable plaque formation in early stage coronary atherosclerosis not detectable by CA. MetSyn needs to be detected and treated as early as possible. Further, hypoadiponectinemia was an important factor for vulnerable plaque formation, particularly plaque volume and calcified lesion into plaque. Measurement of serum adiponectin concentration and appropriate treatment would prevent acute coronary syndrome.
Multiple Roles of Adiponectin from the Progression of Coronary Atherosclerosis to Ventricular Remodeling and Heart FailureMasafumi KitakazeNational Cardiovascular Center
|
Visceral obesity is of increasing interest as a contributor to atherosclerosis, through adipocyte dysfunction leading to the metabolic syndrome (MetSyn). Dr. Masafumi Kitakaze of the National Cardiovascular Center (NCVC) in Suita, Japan and colleagues investigated the predictive role of plasma adiponectin levels in the setting of MetSyn on the progression of coronary artery disease (CAD) and heart failure.
The study population comprised 2,000 subjects in the long-term Nishiarita cohort study and 500 patients with chronic heart failure in the NCVC cohort study. The prevalence of the MetSyn in the Nishiarita cohort study was 5.6% in women and 16.3% in men.
|
||
|
MetSyn is tightly linked to decreases in plasma adiponectin (PA) levels and to atherosclerosis. The downregulation of adiponectin appears to be a key factor driving CAD, as suggested by their finding of significantly higher PA levels in the patients without CAD compared to those with CAD in the NCVC population.
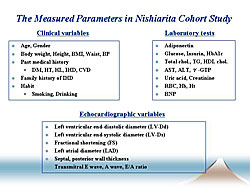
To determine possible new risk factors, this group sought to determine which lifestyle factors contribute to decreased PA levels in the Nishiarita cohort study population. The clinical variables and laboratory tests are shown in Figure 1.
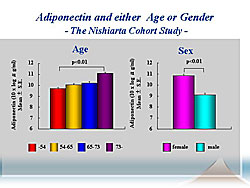
PA levels increased with age and were higher in women compared to men (Figure 2).
They found a relation between a decrease in PA level and an increase in:
• BMI
• waist circumference
• diastolic (but not systolic or mean arterial) blood pressure
• uric acid
• triglycerides
• fasting blood glucose
• HgbA1c
• Insulin level
• HOMA-R index
• ALT or gamma GTP
• Renal dysfunction
• Anemia
• Habitual smoking and alcohol consumption
|
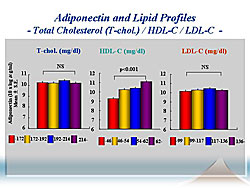
An increase in HDL increased PA levels (Figure 3) Multivariate analysis revealed that age, visceral obesity, BNP, insulin resistance, renal dysfunction, HDL-C, triglycerides, and anemia independently regulate PA levels. Notably, plasma BNP was tightly related to PA levels.
Further, the found a link between PA levels and plasma BNP, fractional shortening and left ventricular diastolic dimension in both populations. The correlation was tightest between BNP and PA levels, suggesting a tight link between them. They also found a tight link between BNP and PA levels, regardless of the cardiac condition.
An in vitro study performed by this group to determine how BNP regulates adiponectin production showed a dose-dependent relation between the expression of adiponectin at 6-12 hours after adipocytes are exposed to BNP. This expression was blocked by an antagonist of a BNP receptor.
To determine the importance of adiponectin in cardiac function, they performed studies in using adiponectin-deficient mice. Aortic-banding induced pressure overload caused worse hypertrophy and heart failure and a higher heart rate in the knockout versus the wild-type mice. Worse fractional shortening and ejection fraction and higher mortality was found in the knockout mice. These finding suggest adiponectin is important for the prevention of cardiac hypertrophy and heart failure.
In conclusion, Kitakaze stated that age, sex, waist circumference, plasma BNP levels, HDL-C, triglycerides, and hemoglobin levels affect PA levels, while plasma insulin, gamma-GTP and creatinine levels had a marginal effect in Nishiarita cohort study.
Intriguingly, the plasma BNP levels were tightly linked to the PA levels in both populations they studied. BNP directly affected adiponectin production in adipocytes in vitro, and more severe cardiac dysfunction was found in adiponectin-deficient mice.
Physiological Roles of a Multi-Ligand Receptor CD36 in the Metabolic Syndrome and Atherogenesis—Insights from CD36 DeficiencyShizuya YamashitaOsaka University Graduate School of Medicine
|
|
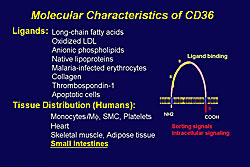
The molecular characteristics of CD36 are shown in Figure 1. Dr. Shizuya Yamashita of Osaka University Graduate School of Medicine reviewed studies performed by his group to elucidate the role of CD36 in metabolic syndrome and cardiovascular disease (CVD).
Five mutations of the CD36 gene have been identified, and it is the only known human genetic deficiency among scavenger receptor families. CD36 deficiency is relatively frequent in the Asian and African populations, with a prevalence of 0.3 % in the Japanese general population. CD36 is a cell transporter of long chain fatty acids (LCFA) in the heart, a major source of energy for cardiac muscles, and its oxidation is important for achieving maximum contractility. About 50% of oxidized-LDL binding is attributed to CD36, according to work by Yamashita and colleagues.
The radioactive 123BMIPP [iodine-123 15-(p-iodophenyl)-(R, S)-methypenta-decanoic acid] is used as a diagnostic tool for evaluating cardiac LCFA metabolism Yamashita and colleagues reported that in control patients there is BMIPP accumulation in the heart, but not in CD36-deficient patients, in whom it accumulates mostly in the liver. Also in CD36-deficient patients, BMIPP uptake is greatly reduced, but it is enhanced in the liver, perhaps related to other transporters of LCFA in the liver.
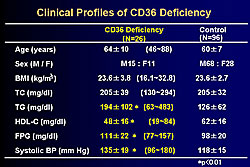
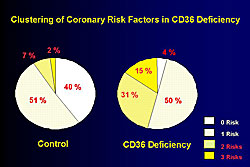
The clinical profile of the CD36-deficient patients studied by this group is shown in Figure 2. CD36 is distributed in lipocytes, however, there is no reduction in body mass index (BMI) and total cholesterol is similar in the patients and controls. However, a significant increase in the patients versus controls was seen for triglycerides, fasting blood glucose, and systolic blood pressure, while HDL-C was lower in the patients than in controls. As shown in Figure 3, 50% of CD36-deficient patients have ≥2 risk factors, including impaired glucose tolerance, hypertension, and hyperlipidemia, compared to only 7% of the controls.
|
|
|
||
|
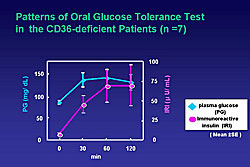
CD36-deficient patients were shown to be insulin resistant by oral glucose tolerance testing (Figure 4) and glucose clamp study. They were also shown to have accumulation of remnant lipoproteins, and IDL-cholesterol was significantly increased in CD36-deficient patients, ranging from 8.5 to 18.5 mg/dL, compared to 3.8 mg/dL in controls.
Oral fat loading testing showed that triglycerides were markedly increased and maintained at 6 hours and ApoB48 was markedly increased in CD36-deficient patients compared to controls. A similar pattern was seen in CD36 knockout mice compared to wild type mice. These data suggest that CD36 is very important in relation to post-prandial hyperlipidemia.
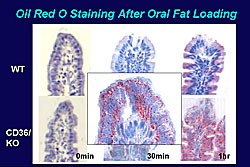
Other work in mice showed that triglyceride levels in the intestinal lymph fluid is increased just a little in wild type mice, but markedly increased in the knockout mice at 3 hours. Further, 3 hours after oral fat loading, chylomicrons, a monitor of triglycerides, was markedly increased in the knockout but not the wild type mice. On oil red staining, there was a marked increase at only 30 minutes of lipid droplets in the knockout mice and at 1 hour triglycerides were greatly increased in the intestinal epithelium; little change was seen in the wild type mice (Figure 5). Oversynthesis of chylomicrons may explain their abnormal increase in the small intestine.
|
Yamashita and colleagues determined that 15 of the 30 CD36-deficient patients studied had coronary heart disease (CHD) and that 8 of the 30 had cardiomyopathy, while none of the controls had either disease. They also found that CD36-deficient patients were 3-times more likely to have CHD than the general population (0.9% vs 0.3%, respectively).
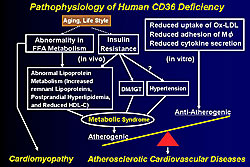
In conclusion, Yamashita reviewed the known pathophysiology of CD36 deficiency and how it contributes to cardiomyopathy and atherosclerotic CVD (Figure 6).
Remnant Lipoproteins, Strong Key Molecules to Atherogenesis in the Metabolic SyndromeYoshio FujiokaKobegakuin University
|
In the metabolic syndrome, several mechanisms contribute to dyslipidemia, notably, triglyceride overproduction and reduced lipolysis associated with reduced lipoprotein lipase activity. This can result in a delay in the clearance of remnant lipoproteins (RLP), evidenced by mixed hyperlipidemia in the setting of diabetes, impaired glucose tolerance, and metabolic syndrome (MetSyn).
RLP play a critical role in atherogenesis, through lipid supply and by inflammatory and post-thrombotic effects, suggesting that RLP are a key molecule for coronary artery disease (CAD) in the MetSyn. Dr. Yoshio Fujioka of Kobegakuin University reviewed data from his group and others that support this finding.
Data from Fujioka’s group and from the Framingham Heart Study show that RLP levels are higher in patients with the MetSyn, and notably it is an independent risk factor for CAD. Nakamura and colleagues recently demonstrated a higher risk for CAD in Japanese patients with MetSyn and elevated levels of hsCRP and RLP. Fujioka and colleagues showed that despite normal cholesterol levels, patients with impaired glucose tolerance or diabetes have higher levels of RLP.
|
Fujioka reviewed experimental evidence of the role of RLP in atherogenesis. Chylomicron remnants are affected by atherosclerosis, and can penetrate the vascular wall and be retained (Figure 1). Further, Abe et al and Doi et al have shown that they can produce adhesion molecules and chemoattractants. They can also lead to foam cell formation of macrophages. Fujioka and colleagues showed that chylomicron remnants can induce MCP-1 expression in vascular smooth muscle cells; that they stimulate the release of IL-1beta by THP-1 cells; and stimulate Egr-1 expression in vascular smooth muscle cells. Recently, Fujioka and colleagues demonstrated that chylomicrons upregulate CD40 expression in monocytes and macrophages (in press). They also had demonstrated these remnants stimulate production of plasminogen activator inhibitor-1 (PAI-1). Apoptosis in vascular endothelial cells induced by chylomicron remnants was also demonstrated by Fujioka and colleagues.
|
|
|
Copyright © 2006 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |