|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
|
|
|
Relationship between Ca2+ Transients and Contractile Force with Elevation of [Ca2+]o The Ca2+-sensitive bioluminescent protein aequorin used for the experiments is an apo-protein extracted from the tentacles of a species of jellyfish, Aequorea aequorea. Aequorin binds three molecules of calcium ions, which leads to bioluminescence that can be used as an index of intracellular calcium concentration in various types of cell, including cardiac and skeletal muscle cells. The rate of light emission is sufficiently rapid to follow the alteration of intracellular calcium concentration, and thus can be used as an indicator of the intracellular calcium concentration in intact myocardium. Alterations induced by changing extracellular calcium concentration is considered to be the standard to compare with those produced by various interventions because only the physiological modulation of the calcium sensitivity induced by the feedback mechanism may contribute to the force-[Ca2+]i relationship. A step-wise elevation of extracellular calcium concentration is accompanied by a parallel increase in the amplitude of calcium transients and contractile force. |
|
|
Intrinsic Regulatory Mechanisms Frank-Starling’s Law The Frank-Starling Law is a measure of myocardial contractility, in which an increase in contractile force induced by stretch of cardiac myocytes reflects well the state of cardiac contractility, and has been established as the length-tension relationship in isolated myocardial preparations and as the ventricular function curve in the whole heart of experimental animals and humans. In the experiment by Endoh and colleagues, it is shown that when the contractile force is markedly altered, depending on the extent of stretch isolated cardiac tissues, there is an essentially unaltered amplitude of calcium transients, which implies that the Frank-Starling’s law is produced by modulation of myofilament calcium sensitivity. The duration of aequorin signal at Lmax is slightly shorter than that at slack length, probably because of an increase in calcium binding affinity by force development at Lmax. The ventricular function curve represents the cardiac pump function, which is at a super-normal state in hyper-thyroidism and is aggravated depending on the extent of the severity of contractile dysfunction, including acute and chronic congestive heart failure (CHF), and in acute circulatory failure (shock).
Subcellular Mechanism of Frank-Starling’s Law Two theories have been proposed to be underlying the Frank Starling mechanism. First, it has been long believed that the optimum overlapping of thick (myosin) filaments and thin (actin, tropomyosin, troponin complex) filaments induced by muscle fiber stretch may be responsible for the development of contractile force. However, more recently it has been proposed that the abbreviated distance between thick and thin filaments caused by the stretch plays a key role in Frank-Starling’s law. Digitalis has been shown to improve the Frank-Starling mechanism in chronic CHF. This shows that the upstream mechanism plays a crucial role for improvement of contractile dysfunction or Frank-Starling mechanism. The subcellular mechanism underlying the most important intrinsic contractile regulation Frank-Starling’s law has not been elucidated unequivocally and awaits further study.
Force-Frequency Relationship In mammalian ventricular myocardium an elevation of heart rate results in an increase in contractile force, which is recognized as a force-frequency relationship, namely a positive staircase phenomenon, which is another important intrinsic regulatory mechanism. In contrast to the Frank-Starling mechanism, the force-frequency relationship is associated with a marked increase in the amplitude of calcium transients. When the frequency of contraction is increased step-wise, the amplitude of calcium transients is increased markedly. The positive force-frequency relationship disappears or is inverted to a negative force-frequency relationship in the late stage of congestive heart failure.
Subcellular Mechanism of Force-Frequency Relationship It is considered that at least two mechanisms are responsible for the positive force-frequency relationship. First, the prolongation of the depolarization duration per unit of time at high stimulation frequency leads to an increase in voltage-dependent L-type calcium channel opening time. Second, the increase in the depolarization frequency leads to an increase in sodium channel opening frequency and thereby causing accumulation of intracellular sodium and thereby increasing intracellular calcium via sodium-calcium exchanger (NCX). The dysfunction of force-frequency relationship in CHF patients has been shown to improve with forskolin, which indicates the important role of the upstream mechanism in the induction of positive force-frequency relationship. |
|
|
Regulation of Ca2+ Signaling via Adrenoceptor Activation
β-Adrenoceptor Stimulation Beta receptor stimulation is the most important external physiological regulatory mechanism, but it is disrupted in CHF. The amplitude of calcium transients is increased to a much higher level in response to isoproterenol, a beta receptor agonist, than in response to increased extracellular calcium concentration. The relation between the developed force and the amplitude of calcium transients during beta receptor stimulation is shifted to the right of the relation with an elevation of extracellular calcium concentration, which may be partly ascribed to a decrease in myofilament calcium sensitivity in relation to phosphorylation of troponin I and an abbreviation of calcium transients produced by activation of SERCA2a due to phosphorylation of phospholamban (PLB) induced by beta receptor stimulation.
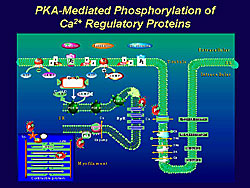
Abbreviation of twitch contraction was much more pronounced than that of calcium transients, as shown in work by Endoh and colleagues in aequorin-loaded rabbit papillary muscle, which implies that the significant contribution of a decrease in myofilament calcium sensitivity to acceleration of cardiac relaxation induced by beta receptor stimulation. The subcellular mechanisms of the characteristic effects of beta stimulation are ascribed to the phosphorylation of regulatory proteins, such as L-type calcium channel, ryanodine receptor, phospholamdan, troponin I, and myosin binding protein C (Figure 1). More recently, it is shown that the NCX is also phosphorylated by activation of PKA.
α-Adrenoceptor Stimulation Regulation induced by alpha receptor stimulation contrasts strongly with the beta- receptor mediated regulation. In the rabbit papillary muscle, for a given (equivalent) increase in contractile force, the increase in calcium transients with alpha receptor stimulation induced by phenylephrine in the presence of the beta-blocker bupranolol was much lower than that induced by the elevation of the extracellular calcium concentration. Alpha stimulation increases myofilament calcium sensitivity in addition to a moderate increase in calcium transients. The duration of twitch contraction is prolonged, while the duration of calcium transients is reciprocally abbreviated, indicating an increase in myofilament calcium sensitivity induced by cardiac alpha receptor stimulation. The increase in calcium transients induced by alpha receptor stimulation may be ascribed to a direct activation of L-type calcium channel or an indirect increase induced by prolongation of action potential duration due to suppression of potassium channels, and in part due to activation of NCX subsequent to intracellular sodium accumulation elicited the activation of the sodium-hydrogen exchanger (NHE). It is noteworthy that some processes activated by alpha stimulation lead to a decrease in intracellular calcium concentration: activation of sodium-potassium ATPase and/or activation of potassium channels that results in an abbreviation of action potential duration. These effects counteracting the positive inotropic effect of alpha stimulation may contribute to a wide range of species dependent variation of cardiac alpha receptor regulation. The increase in myofilament calcium sensitivity induced by alpha stimulation may be partly due to intracellular alkalinization subsequent activation of NHE. Although alpha stimulation promotes the phosphorylation of troponin I and troponin T via activation of PKC, its functional relevance of, however, has not been established probably due to variations of PKC isoforms involved in the regulation. More recently, it has been proposed that phosphorylation of myosin light chain 2 (MLC-2) may be responsible for the increase in calcium sensitivity induced by cardiac alpha receptor stimulation. |
||||
|
Calcium Sensitizers Acidosis and Mechanisms of Action of Cardiotonic Agents
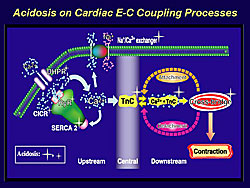
Cardiac contractile function and the effects of cardiotonic agents are markedly influenced by acidosis that occurs in acute heart failure, namely during ischemia-reperfusion (Figure 2). While it has been shown that various functional proteins involved in cardiac calcium signaling are affected by acidosis, experiments by Endoh and colleagues indicate that the decrease in calcium binding affinity of troponin C is the most prominent feature affected by acidosis in intact ventricular myocardium. When the extracellular pH is lowered in the physiological solution from 7.4 to 6.6, there is a marked decrease in twitch contraction, but the amplitude of calcium transients is further increased, and there is a complete dissociation between calcium transients and contraction. This implies that calcium mobilizers lose their effectiveness in the setting of acidosis.
Org 30029 Org 30029 is a highly effective calcium sensitizer. In the experiment carried out by Endoh and colleagues in aequorin-loaded canine ventricular myocardium, this compound was shown to increase the contractile force to a much higher level (150% of ISOmax) than the maximum effect achieved with isoproterenol. Further, Org 30029 elevated calcium transients only to a small extent and prominently increased the contraction duration. The calcium sensitizing action of Org 30029 is not affected by acidosis or by BDM, which has been known to cause uncoupling of force development from calcium transients. These observations imply that Org 30029 is able to elicit its cardiotonic effect even under acidosis, and even when the effectiveness of calcium mobilizers is markedly attenuated.
Levosimendan and Its Active Metabolite OR-1896
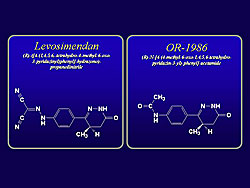
The chemical structure of these compounds is shown in Figure 3. Levosimendan has been selected from compounds that bind to troponin C, and has been shown to increase calcium sensitivity with a high affinity by stabilizing the structure of the calcium-troponin C complex. Under both normal and acidotic conditions, levosimendan exhibits a dual action on calcium signaling in the work by Endoh and colleagues in aequorin-loaded canine ventricular muscle. Contracile force increases in association with a moderate increase in calcium transients at concentration range up to10-5 Molar. However, at a concentration of 10-4 Molar, levosimendan decreased the contractile force markedly in association with a further increase in calcium transients, indicating that it decreases myofilament calcium sensitivity at higher concentrations. Levosimendan increases contractile force and calcium transients over the same concentration range up to 10-5 Molar. At higher concentrations, the contractile force is decreased markedly, whereas the level of calcium transients is maintained high. The positive inotropic effect of levosimendan is associated with a slight but significant abbreviation of the duration of contraction. Notably, a negative inotropic effect occurs with levosimendan at concentrations greater than 3 x 10-5 Molar, with a further increase in calcium transients. Levosimendan is shown to elicit a positive inotropic effect even under acidotic conditions in the canine ventricular muscle. While the calcium sensitizing effect of levosimendan is maintained even under acidosis, its efficacy is attenuated because of a decrease in the amplitude of calcium transients induced by the compound under acidotic conditions. The influence of acidosis on the positive inotropic effect of the active metabolite OR-1896 was very similar to that of the mother compound levosimendan, except that OR-1896 did not elicit a negative inotropic effect, even at higher concentrations. The calcium sensitizing effect of OR-1896 was not suppressed under acidosis, but because the increase in calcium transients induced by OR-1896 is markedly attenuated under acidosis, the positive inotropic effect of OR-1896 was suppressed under acidosis, even though the calcium sensitizing effect of the compound was not suppressed by acidosis.
Differential Effects of Acidosis on Calcium Sensitizers
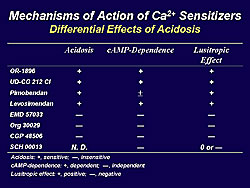
The differential effects of acidosis on various calcium sensitizers are shown in Table 1. Calcium sensitizers are classified in to two groups depending on the susceptibility to acidosis. These is a clear tendency that the positive inotropic effect of calcium sensitizers that act via downstream mechanism are resistant to acidosis, while some agents acting through central mechanism are likewise less susceptible to acidosis.
Cardiotonic Agents and Congestive Heart Failure The efficacy of cardiotonic agents is markedly affected in chronic CHF. Work in an animal model of volume-overload CHF by Endoh and colleagues provides experimental evidence for differential modulation of the positive inotropic effect of the calcium mobilizer and calcium sensitizer in the chronic congestive heart failure model. In volume-overloaded rabbit heart with an A-V shunt, at 12 weeks myocardial hypertrophy and pleural effusion were present, indicating the presence of chronic CHF. Significant fibrosis in the left ventricular papillary muscle was seen at 4 weeks (8.5%) and 12 weeks (15.2%) compared to the sham animal (1.6%). Ventricular cardiomyocytes were significantly longer in the animals with CHF compared to the sham animals. Cell shortening and the indo-1 ratio in response to isoproterenol and dobutamine were significantly attenuated in ventricular cardiomyocytes isolated from the animals with CHF compared to sham animals, whereas the positive inotropic effect of the calcium sensitizer EMD 57033 was not affected at all by CHF compared to control animals. The observations indicate that the effect of certain calcium sensitizers is essentially unaltered in volume-overloaded cardiomyocytes, whereas the effectiveness of calcium mobilizers that act by upstream mechanisms is attenuated.
Therapeutic Relevance of Ca2+ Sensitizers The therapeutic advantages of calcium sensitizers include the lack of risk of calcium overload resulting in arrhythmogenicity and myocardial cell injury and death. There are energetic advantages such that there is no increase in activation and metabolic energy. Furthermore, calcium sensitizers are effective even under pathological conditions, such as acidosis, stunned myocardium, and CHF, and they can be used with digitalis without arrhythmogenesis. A potential disadvantage may be diastolic dysfunction due to an increase in calcium sensitivity to the calcium concentration at diastolic level. Weak PDE 3 inhibitory action associated with certain calcium sensitizers such as pimobendan and levosimendan may counteract this potential risk.
Conclusions The upstream mechanism of calcium mobilization plays a primary role in contractile regulation by β-stimulation and force-frequency relationship. Central and/or downstream mechanisms (calcium sensitization) play a crucial role in contractile regulation, via the Frank-Starling mechanism, α-stimulation, and calcium sensitizers. Calcium sensitizers act via different subcellular mechanisms and have a differential response in the setting of acidosis. Calcium sensitizers, namely those that act via downstream mechanism on the crossbridge, possess a high potential for pharmacotherapy of cardiac contractile dysfunction in CHF patients. |
||||||||||
|
|
|
Copyright © 2007 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |