|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
State of the Art: Pharmacogenomics of Heart FailureArthur M. FeldmanThomas Jefferson University, Philadelphia, USAClinical trials have shown that not all heart failure patients respond to pharmacologic therapy. Nor have they demonstrated a correlation between patient phenotype and response to any given drug. Recent studies, reviewed by Dr. Arthur M. Feldman, Thomas Jefferson University, show that an individual’s genotype can predict response to heart failure drugs.
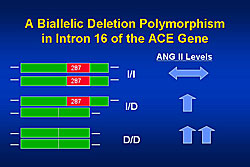
In 1990, the CONSENSUS trial reported that heart failure patients with higher levels of angiotensin II, norepinephrine, or aldosterone had higher mortality than those with lower levels (p<0.001). Analysis of ACE genotypes revealed that patients with the wild type ACE gene (ACE I/I) have normal angiotensin II levels, while patients with a deletion in both alleles (ACE D/D) have far higher levels, and those with a single allele deletion have intermediate levels (Figure 1). The presence or absence of these genes is not associated with incidence of heart failure. However, follow-up of heart failure patients over time has shown that patients with the ACE I/I gene have a longer transplant free survival than those with the ACE I/D or DD gene (p=0.04). In the GRACE trial of low-dose ACE inhibitors, patients with the I/I gene had a much better response and higher event-free survival than those with the D/D gene (p=0.005). This difference in outcomes was not observed in patients receiving high-dose ACE inhibitors.
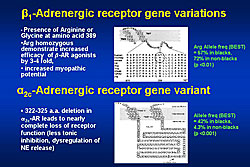
Two adrenergic receptor gene variants are important with respect to norepinephrine release and response to beta-blockers (Figure 2). The BEST trial studied the beta-blocker bucindolol versus placebo in a population enriched for women and minorities, from whom genetic material was prospectively collected. Overall, there was no survival difference between the two treatment groups, but patients with homozygous Arg 389 receiving bucindolol had better survival than those receiving placebo (HR=0.62, p=0.030). Increased aldosterone levels are associated with an aldosterone synthase promoter polymorphism at position -344. While the normal genotype is -344 TT, a C shift can occur on one (-344 TC) or both (-344 CC) alleles. No difference in survival was found between these genotypes in a heterogeneous population with heart failure. The GRACE and GRAHF trials found a lower incidence of the C allele in the African-American subset (A-HeFT) than in white Americans. However, the African-American patients with the CC gene had a much worse prognosis than those with the TT gene (p=0.018). Further, patients with the TC or CC gene had no response to treatment with isorsorbide-dinitrate therapy. Dr. Feldman concluded that pharmacogenetics is the future of therapy for patients with heart failure. This approach will allow prospective individualized targeting of cardiovascular drugs. |
Statin Pharmacogenomics in Heart Failure Treatment: Lessons from Coronary Heart Disease PreventionKouji KajinamiKanazawa Medical University, UchinadaEvidence from recent studies suggests potential clinical applications for statins in the prevention of coronary artery disease (CAD). The pleiotropism of statins on endothelial function, inflammation, and oxidative stress may also lead to statin therapy for prevention of heart failure. Dr. Kouji Kajinami, Kanazawa Medical University, described pharmacogenomic studies his group conducted to explore genetic factors influencing statin therapy in CAD prevention. The study outcomes were lipid response and cardiovascular event response.
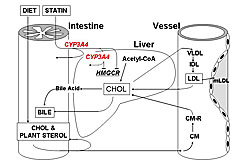
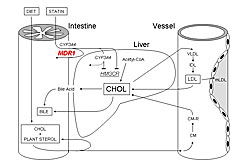
To determine genetic influence on statin pharmacokinetics, atorvastatin response was studied in patients with different CYP3A4A-290G and multidrug resistance-1 (MDR1) C3435T genotypes. After therapy, patients with the CYP3A4A-290G GG variant had significantly higher LDL cholesterol (LDL-C) than those with AA or AG genotypes (p=0.038). This difference appears to be associated with greater CYP3A4 expression in GG homozygotes, resulting in less atorvastatin reaching the liver and decreased LDL-C reduction (Figure 1). Comparison of MDR1 C3435T genotypes showed a significantly greater reduction from baseline in LDL-C (p=0.023) and triglycerides (p=0.0313) in women with TT and CT variants compared to wild-type (CC). This difference was not observed in men. These variants were shown to reduce MDR1 activity (Figure 2).
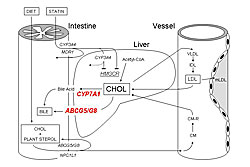
Other studies examined pharmacodynamic targets, including intestinal cholesterol absorption (ABCG8), cholesterol production (CYP7A1), and lipoprotein catabolism (APOE). Patients with the ABCG8 DH/HH genotype had a significantly greater response to atorvastatin than DD homozygotes in LDL-C (p=0.028) and triglyceride (p=0.012) lowering. Patients with wild-type CYP7AA1 had greater cholesterol reduction than those with CYP7AA1 Ht or Hm variants. A study of interactions between CYP7A1 and ABCG8 showed that patients with an ABCG8 variant and CYP7A1 wild-type genotype had the greatest cholesterol lowering. The mechanisms of these effects are shown in Figure 3. APOE variants also have different effects on response to atorvastatin, which are increased when analyzed in combination with CYP7A1 variants (wild-type CYP7A1 plus APOE E3 results in greatest LDL-C reductions). In these and other studies, the absolute difference in LDL-C reductions across genotypes has been 3-6%. Combined analysis of multiple variants in several genes produced greater absolute differences of 8-10%. Dr. Kajinami concluded that these studies provide data that could lead to similar studies for therapy of heart failure with statins, resulting in more individualized management. |
|
|
|
Copyright © 2007 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |