 |
|
|
 |
| Angiogenic Therapy
for Coronary Artery Disease |
|
|
Hiroki Yokomuro, M.D.
Toho University,
Tokyo, Japan
Hidezo Mori, M.D.
National Cardiovascular
Center, Research Institute, Suita, Japan
Hiroshi Kamihata, M.D.
Kansai Medical
University, Moriguchi, Japan
Yoshiki Sawa, M.D.
Osaka University,
Osaka, Japan
|
|
|
|
 |
|
|
 |
|
Transplanted
Cryopreserved Cardiomyocytes |
|
The transplantation of cardiomyocytes has been shown
to be an attractive and useful strategy for cardiac
functional improvement after myocardial damage by
Yokomura and colleagues at Toho University. This group
also demonstrated that cardiomyocytes can be cryopreserved.
In the present study, they evaluated whether transplanted
cryopreserved rat cardiomyocytes would survive in
the connective tissue of the hind limb in the adult
rat.
|
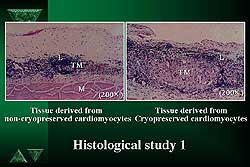
 |
| Figure
1. No differences were seen between the cryopreserved
and non-cryopreserved cardiomyocytes on histology.
|
| Click
to enlarge |
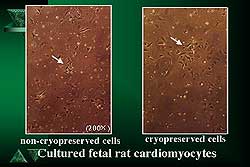
 |
| Figure
2. Microscopy revealed no differences between
the cryopreserved and non-cryopreserved cardiomyocytes.
|
| Click
to enlarge |
|
Cryopreserved samples (using a freezing medium to
reach minus 80 degrees Celsius, then stored in liquid
nitrogen for 1, 2, 4, 8, 12 and 24 weeks) were shown
on histology after thawing to look quite similar to
non-cryopreserved cells, with no morphological differences
(Figure 1). Figure 2 shows microscopic images of cryopreserved
and non-cryopreserved cardiomyocytes. No differences
were observed in cell proliferation at each time point,
and cell proliferation gradually decreased at the
same rate in the cryopreserved and fresh cardiomyocytes
over time. No difference was observed in the percentage
of cryopreserved beating cells compared to fresh cells
at each time point, and the percentage gradually decreased
in both groups over time.
An in vivo study was then conducted using the same
methodology, except the cells were cryopreserved for
one week. Cardiomyocytes were injected using a tuberculin
syringe into the subcutaneous tissue of the adult
rat. Cyclosporin A (5 mg/kg) was administered subcutaneously
daily. The rats were sacrificed at 4 weeks after transplantation.
Survival and contractility of the transplanted tissue
were evaluated visually, histologically, and by electrocardiogram.
The electrical activity of the recipient heart was
about 219 beats per minute (bpm), while the electrical
activity of the cardiac-like tissue formed from transplanted
cryopreserved cardiomyocytes was 60 bpm. On histology,
the non-cryopreserved tissue size was smaller and
infiltration of lymphocytes was similar to that of
cryopreserved cells. New blood vessel-like tissue
was observed in the transplant region.
The function and morphology of tissue derived from
cryopreserved cardiomyocytes and the cultured cardiomyocytes
were similar to that of non-cryopreserved cells, stated
Hiroki Yokomuro, MD. Transplanted cryopreserved cardiomyoctyes
survived and contracted spontaneously in connective
tissue. Cryopreservation likely affected the reduction
of immunogenecity for transplanted cells. The investigators
concluded that this storage technique may be applied
to future cell transplantation.
|
PAGE
TOP
|
Gene Therapy
with Biodegradeable Gelatin Hydrogel |
|
Gene transfer by viral vectors is an efficient but
biohazardous process. Naked DNA transfer, although
safer, is less efficient. Mori and colleagues demonstrated
that angiogenic gene therapy can be potentiated using
biodegradeable gelatin hydrogel (GHG). A non-viral,
but highly efficient gene therapy for salvaging ischemic
rabbit hindlimb was achieved with fibroblast growth
factor-4 (FGF4)-GHG (FGF4-GHG). The degree of development
and maturity of the therapeutic angiogenesis were
evidenced by synchrotron radiation (SR) microangiography.
In 6 transfected rabbits, FGF4 transgene expression
was evaluated on day 17 of hindlimb ischemia. RT-PCR
analysis revealed FGF4 transgene expression in all
injection sites with naked FGF4 and FGF4-GHG. However,
expression was detected 10 mm apart from the injection
site in the FGF4-GHG-treated animal, but not in the
naked FGF4-treated animal.
Then one of three gene therapies was performed 10
days after femoral artery resection: 1) LacZ 500mcg
+ GHG (n=8), 2) naked FGF4 500 mcg (n=7), and 3) FGF4
500 mcg + GHG (n=7). On day 38, conventional angiography,
SR microangiography, gross anatomical and molecular
biological studies were performed.
Severe toe necrosis and thigh muscle atrophy and
necrosis were noted in the control rabbit treated
with LacZ-GHG. In contrast, ischemic tissue damage
was nearly negligible in FGF4-GHG-treated rabbits.
Severe tissue damage was noted in the LacZ-GHG rabbit,
less damage in the naked FGF4 rabbit, and the least
tissue damage in the FGF4-GHG rabbits. All of the
differences were statistically significant.
Conventional angiography showed that in the naked
FGF4 and FGF4-GHG-treated rabbits that the midzone
collaterals developed substantially. However, no statistical
difference was seen between the groups. SR microangiography
showed that flow reserve is preserved in the FGF4-GHG
rabbit, while flow still phenomenon developed in the
naked FGF4 rabbits, demonstrating a greater degree
of angiogenesis in the FGF4-GHG rabbits. A statistically
significant difference between the groups was shown
by the angiographic score ratio.
In a subsequent study using the same protocol in
the canine model, these investigators demonstrated
significantly enhanced fractional shortening and higher
flow reserve in the FGF4-GHG-treated animals, compared
to LacZ-treated animals.
|
PAGE
TOP
|
Autologous
Bone Marrow Implantation |
|
Bone marrow (BM) is a natural source of endothelial
progenitor cells (EPC) and a broad spectrum of cytokines.
Bone marrow-mononuclear cells (BM-MNC) containing
EPC are mobilized from BM in response to tissue ischemia
or VEGF therapy to accumulate in ischemic lesions.
Autologous
implantation of BM-MNC markedly augmented new capillary
formation in ischemic myocardium, resulting in increased
regional blood flow and improvement in cardiac function
in a study conducted by Kamihata and colleagues at
Kansai Medical University. BM-MNC synthesized and
secreted angiogenic ligands such as basic fibroblast
growth factor (bFGF), vascular endothelial growth
factor (VEGF) and angiopoiten-1. BM-MNC implanted
in ischemic myocardium was incorporated into capillary
vessel walls, stated Kamihata.
BM-MNC derived from mini-swine ileum were injected
into the ischemic zone. A control group was injected
with medium alone into the ischemic zone after irradiation.
Angiogenesis was evaluated at week 3. The myocardium
was removed for assessment of the infarcted area and
imunohistochemistry.
The distal portion of the LAD was visible in all
animals on coronary angiography. However, the number
of visible collateral vessels branching from the left
circumflex coronary artery in the direction of the
infarct tended to increase in the BM-MNC-implanted
animals compared to the control animals.
A perfusion defect was seen at week 3 in the control
animals on MCE using second Harmonic technology, which
was significantly different from baseline, and a persistent
flow deficit in the ischemic region. In the BM-MNC-implanted
animals, the perfusion defect was markedly reduced,
as much as 83% compared to the baseline value.
The LVEF was significantly improved by 48% in the
BM-MNC-implanted group at 3 weeks after irradiation,
while it was decreased by 11% in the control animals
from baseline values. The extent of the maximum left
ventricle DPTT deterioration was less in the BM-MNC-implanted
group compared to the control group.
The number of capillaries in the ischemic portion
increased about 2.5-3.0-fold in the BMI group, compared
to control as seen on immunohistochemistry. Analysis
revealed that 34% of vessels incorporated BM-MNC.
Since not all cells incorporated MNC, the investigators
hypothesized that the MNC may release angiogenic factors
in transplanted myocardium to enhance angiogenesis.
On further examination, they found that MNC expressed
more mRNA for bFGF than for VEGF and angiopoietin-1,
but not greater than angiopoieten-2.
Autologous bone marrow implantation may constitute
a novel a strategy for achieving optimal therapeutic
angiogenesis, concluded Kamihata, by exploiting the
natural ability of bone marrow cells to secrete important
angiogenic factors and its ability to incorporate
into foci of neo-vascularization.
|
PAGE
TOP
|
Hepatocyte
Growth Factor Gene Therapy |
|
Intramyocardial transfection of genes encoding growth
factors such as VEGF constitutes an alternative strategy
for patients with severe myocardial ischemia. Two
clinical reports have shown the beneficial effect
of VEGF DNA for therapeutic angiogenesis. Hepatocyte
growth factor (HGF) has been postulated as a potential
growth factor, with potential cell protective functions
and angiogenesis, apoptosis, and anti-fibrosis properties
with a specific HFG receptor (c-Met). However, the
role of HGF in the ischemic heart, especially its
role in angiogenesis, has not been clarified.
Sawa and colleagues at Osaka University have
reported that HGF and c-Met is upregulated in ischemic
myocardium in rat hearts, and is limited to the ischemic
region. Gene transfection of HGF prevents ischemia
and reperfusion injury in rat hearts. This evidence
supports the notion that the HGF/c-Met system may
play a role in the angiogenesis of ischemic myocardium.
In the present study, the effect of direct injection
of plasmid DNA of human HGF as therapeutic angiogenesis
was investigated using the canine heart. Left coronary
angiography revealed that the LAD was supplied with
collateral vessels enhanced by HGF injection. The
capillary count in the ischemic myocardium increased
significantly after direct injection of HGF. Regional
contractile function and blood flow in the ischemic
areas showed significant recovery after HGF treatment.
No significant difference in leukocyte number was
observed between the groups.
Twenty-two canines divided into 3 groups were given
HGF-cDNA (125 mcg injection; Group H), LacZ cDNA (125
mcg injection; Group L) and a sham control group (Group
S). Ligation was performed at the mid-portion of the
LAD, just below the first diagonal branch. After1
month of LAD ligation, direct injection was performed
at six ischemic points.
At 4 days after gene transfection, Group H showed
marked expression of human FGF, while Groups L and
S did not. One month after gene transfection the LAD,
undetectable before transfection, was supplied by
neo-vasculature, i.e., the angiogenic effect of FGF
gene transfection was visible. The number of factor
VIII positive endothelial cells was markedly increased
in Group H compared to Group L on histology. The capillary
density in the ischemic myocardium was significantly
higher in Group H than in the two other groups. The
percentage of thickening fraction was significantly
higher and the regional blood flow in ischemic myocardium
was significantly improved in Group H than in the
other two groups.
VEGF has a potent angiogenic function but it increases
membrane permeability. HGF has angiogenic function
and increases proliferation of endothelial cells,
but not smooth muscle cells. Therefore, these investigators
compared the effect of HGF and VEGF plasmid transfection
using the canine LAD ligation model. The number of
factor VIII positive endothelial cells was markedly
increased in both groups, compared to the LacZ group.
The density of capillaries in the ischemic myocardium
was significantly higher in the HGF and VEGF groups
compared to LacZ and sham groups. Only the VEGF group
showed a higher value in terms of water content, suggesting
increased membrane permeability in this group.
The direct injection of plasmid DNA encoding human
HGF into the ischemic myocardium may be an angiogenic
therapy improving regional perfusion and contractile
function, concluded Yoshiki Sawa, MD. Thus, continuous
local production of HGF may be considered as a novel
therapeutic angiogenesis strategy for ischemic heart
disease, such as myocardial infarction. Based on these
data, they are preparing a protocol for a clinical
trial using HGF.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2001
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|