 |
|
|
 |
| Calcium Cycling Proteins of Cardiac Sarcoplasmic Reticulum (SR): Molecular
Regeneration of the Phospholamban-SERCA Ca Pump System and Its Pathophysiological
Consequences |
|
Michihiko Tada
Osaka University Medical School, Suita, Japan
Genome Analysis and Medical Information Center, Ltd, Hyogo, Japan |
|
 |
|
 |
|
|
 |
|
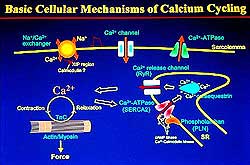 |
| Figure
1. Scheme of the basic cellular mechanisms in
calcium cycling. |
| Click
to enlarge |
|
Two major functional membrane proteins, Ca2+
pump ATPase (SERCA), and phospholamban (PLN) play
major roles in the control of calcium cycling during
excitation-contraction (EC) coupling. This lecture
focused on the Ca pump ATPase/PLN system, important
for muscle relaxation by pumping intracellular Ca2+ions.
Release of Ca2+ from the lumen of sarcoplasmic
reticulum (SR) to the intracellular space caused by
the Ca2+ release channel/ryanodine receptor
triggers contraction. (Figure 1).
Tada reviewed most of the molecular biological
aspects of the PLN-SERCA2 system, which governs the
Ca cycling mechanism underlying the EC coupling of
the myocardium, and the inhibitory effects of PLN
and SERCA2 that can cause a "calcium cycling
defect" that results in cardiac hypertrophy and
even failure. Notably, further understanding of the
data is required to determine causal relationships.
|
|
Molecular Biology of PLN and SERCA |
|
The intracellular interplay between cyclic AMP (cAMP)
and Ca2+ comprises ß-adrenergic stimulation
of cardiomyocytes, after which the ß-receptor/adenylate
cyclase system produces cAMP to activate cAMP-dependent
protein kinase (A kinase) A kinase catalyzes phosphorylation
of at least three functional proteins in cardiac myocytes,
which are essential for Ca cycling mechanisms: the
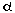 -subunit
of the voltage-sensitive Ca channel; PLN in the SR;
and troponin I (Tn-I) in the myofibrillar apparatus.
Interestingly, the former two increase Ca2+ in
and out of the cytoplasm, while Tn-I phosphorylation
protects myofibrils from overreacting to an increased
flow of intracellular Ca2+. -subunit
of the voltage-sensitive Ca channel; PLN in the SR;
and troponin I (Tn-I) in the myofibrillar apparatus.
Interestingly, the former two increase Ca2+ in
and out of the cytoplasm, while Tn-I phosphorylation
protects myofibrils from overreacting to an increased
flow of intracellular Ca2+.
|
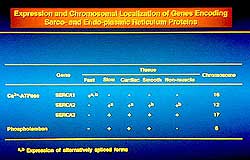 |
| Figure
2. Expression and location of genes encoding SERCA
proteins. |
| Click
to enlarge |
 |
| Figure
3. Major characteristics of phospholamban. |
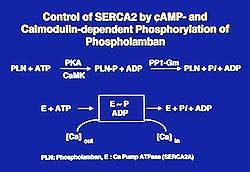 |
| Figure
4. Illustration of the control of SERCA3 by cyclic
AMP and calmodulin-dependent phosphorylation of
phospholamban. |
| Click
to enlarge |
|
Early data from Tada and colleagues identified the
presence of PLN in cardiac SR. The three genes for
Ca2+-ATPase (SERCA, Sarco Endoplasmic Reticulum
Calcium ATPases) are: SERCA1, expressed in fast-twitch
skeletal muscle SR; SERCA2, expressed in cardiac,
slow-twitch skeletal and smooth muscles; SERCA3, expressed
in a variety of muscle SR and non-muscle cell ER and
considered a housekeeping gene Figure 2). PLN and
SERCA2 are always co-expressed, because only one PLN
gene has been identified to date, which is expressed
in the SR of cardiac, slow-twitch skeletal and smooth
muscle cells. SERCA2a is expressed in cardiac and
slow-skeletal muscles and SERCA2b in smooth muscle
and non-muscle cells. Figure 3 summarizes the characteristics
of PLN.
PLN phosphorylation, phosphoester in nature,
occurs at the Ser and Thr residues, whereas the phosphoprotein
intermediate of ATPase is acyl phosphate in nature
and occurs at the Asp residue (Figure 4). CaM kinase
catalyzes phosphorylation of the Thr residue in addition
to the A kinase-catalyzed phosphorylation at the Ser
residue. The formed phosphoprotein is nearly immediately
dephosphorylated by PP1 (protein phosphatase type
1). Tada and colleagues showed that upon PLN phosphorylation,
the rates of formation and decomposition of the ATPase
intermediate E-P are enhanced and thus increase the
turnover rate of the ATPase reaction. The tight coupling
of ATP hydrolysis and Ca2+ translocation
across the SR membrane enhances the rate of Ca uptake
by PLN phosphorylation. The affinity of the Ca pump
for Ca2+ is increased.
Further work by Tada and colleagues identified
4 intermediary steps in the formation and decomposition
of E-P. Of these steps, the rates of conversion from
E2 to E1 and the rate from E1-P
to E2-P are enhanced when PLN is phosphorylated
by A kinase. Similar results were obtained with CaM
kinase phosphorylation of PLN. Hence, they proposed
the working hypothesis that PLN would act directly
on the Ca pump ATPase through a protein-protein interaction.
|
PAGE
TOP
|
PLN-SERCA Molecular Interactions |
|
The molecular mechanisms of protein-protein interactions
between PLN and SERCA2a were explored by Tada and
colleagues via cross-linking experiments employing
the cross-linker Denny-Jaffe reagent, with Iodine-125
(I-125) labeled on one end with an N=N bond and on
the other end Lys protein residues. Figure 5 illustrates
the trial design. Labeled PLN was incubated with purified
SERCA Ca pump ATPase in the presence of a non-ionic
detergent and the two proteins were cross-linked under
UV light. Two conditions were required for cross-linking:
1) PLN in a dephospho state, and 2) Ca pump ATPase
in a Ca2+ -free E2 state. The
double N=N bond was broken by adding sodium dithionite
to obtain I-125-labeled Ca ATPase. The labeled Ca
ATPAse was fragmented by cyanogen bromide to identify
the I-125-labeled ATPase segments. Two significant
features were identified: 1) SERCA1 and SERCA2 have
I-125 labeled sequences at two Lys residues of an
ATPase protein fragment, while SERCA3 does not have
a similar sequence nor is labeled by I-125, and 2)
a sequence similarity between the PLN binding site
located less than 50 amino acid downstream of the
active ATPase site (forming E-P SERCA1, expressed
in fast-twitch skeletal muscle which is devoid of
PLN) and the SERCA2 PLN-binding site, thus indicating
that the differential regulation by PLN is a result
of tissue-specific PLN expression. The amino acid
sequence around the PLN binding region in SERCA isoforms
is shown in Figure 6.
|
|
|
 |
| Figure
6. Comparison of the amino acid sequence around
the PLN binding region in SERCA isoforms. |
| Click
to enlarge |
|
|
Construction of a series of chimeras of SERCA2 and
SERCA3, subjected to assay for determining Ca uptake
rates, provided additional insights of the PLN and
SERCA molecular interactions. Co-expression of PLN
inhibits SERCA2 by shifting the Ca-dependence curve
to higher Ca2+ concentrations, but it has
no effect on SERCA3. Chimera CH2, mostly derived from
SERCA2, does not react to co-expressed PLN, similar
to the sequence data. However, Chimera CH9, mostly
derived from SERCA3 with a PLN-binding sequence derived
from SERCA2, shows no reaction to PLN co-expression,
which differs from sequence data. Further study revealed
that a secondary interaction site in the ATPase protein
within the SR membrane is required for PLN to fully
inhibit the Ca uptake rate.
An electrochemical milieu that allows PLN to
bind to the PLN-binding domain of SERCA2 is established
by the interaction between the cluster of charged
residues (K-D-D-K plus PV in SERCA2) and charged residues
in the PLN domain IA. Incorporation of phosphate into
Ser16/Thr17 in PLN perturbs the charged interactions
by introducing negative charges and results in dissociation
of PLN from SERCA, which allows augmentation of ATPase
activity.
|
PAGE
TOP
|
|
The mutation of transmembrane residues of PLN provided
insights into the molecular interactions between the
TM regions of PLN and SERCA2. Studies showed functional
polarization, i.e., gain of function residues located
in one region and loss of function residues located
in another. This functional polarization is important
in establishing the transmembrane interaction of PLN
and SERCA2, since gain of function mutants of PLN
were about 75% monomeric and 25% pentameric, whereas
the wild-type PLN, the loss of function mutants and
the no change mutants were about 75% pentameric and
25% monomeric. In other words, gain of function mutants
with about a 3-fold increase in their inhibitory function
accompanied the 3- to 4-fold enhancement of monomer
formation. The results indicated that a direct consequence
of monomer formation may be gain of function mutations.
In terms of loss of function mutants, the pentameter
stability remained unchanged compared to wild-type
PLN, suggesting that the interacting surface for SERCA2
lies on one face of the helix. Thus, PLN monomers
are thought to represent the functional form, while
the PLN pentameters provide a reservoir for the active
monomer. This leads to the notion that the key determinant
of inhibitory function must be the concentration of
the PLN monomer/SERCA2 complex formation.
PLN was shown to be directly associated with
the M6 segment of SERCA2. Three of 10 transmembrane
segments of SERCA (M4, M5, M6) were shown to form
a pore for two Ca2+ ions, providing further
evidence that PLN can alter the affinity of SERCA2
for Ca2+ by a direct protein-protein interaction.
Two SR proteins, among others, Ca2+-release
channel/ryanodine receptor (RyR) and SERCA2 plus PLN,
play major roles in controlling the rise and fall
of intracellular Ca2+ to induce contraction-relaxation
cycles. Myofibrillar contraction is initiated by Ca2+-induced
Ca2+-release by the RyR stored in the lumen
of SR, when voltage-sensitive Ca2+ channels
on the sarcolemma open to allow Ca2+ entry
into the cell. Relaxation is caused by the uptake
of Ca2+ through SERCA and PLN. Under pathologic
conditions, when cells are overloaded with Ca2+,
the removal of Ca2+ by the Na+/Ca2+
exchanger is activated to supplement the SERCA2
activity.
Work by other investigators shows the consequences
of PLN/SERCA2 transgenic mutations in vivo.
This works has shown 1) that the ablation of PLN in
a mouse model of dilated cardiomyopathy rescues the
phenotype that resembles human heart failure; and
2) that superinhibition of SERCA2 by PLN mutants causes
cardiac hypertrophy and failure. Work by Tada and
colleagues aims to show that mutation of the PLN-binding
sequence in SERCA2 prevents PLN from forming the active
complex, so that Ca2+ uptake is augmented
to attenuate pressure overload-induced cardiac hypertrophy.
PLN ablation has been shown by other investigators
to rescue macroscopic and ultrastructural defects
of the MLP knockout mice myocardium. In this setting,
Ca signaling defects seen in dilated cardiomyopathy
are rescued when PLN is ablated and restored to the
normal pattern compared to the effects in wild-type
mice. Cardiac contractility is activated by inhibition
of the interaction between SERCA2 and Val49
to ALA mutation of PLN, a loss of inhibitory function
mutation. The data indicate the possibility that PLN
ablation and mutation rescue the "Ca cycling
defect" in dilated cardiomyopathy.
Tada and colleagues showed that a mutant SERCA2
transgene, constructed such that positively-charged
K397 and K400 are mutated to
negatively charged glutamic acids, had a 10-fold greater
affinity for Ca2+ in the transgenic mice,
compared to the wild-type and non-transgenic mice.
Further, in the mutant SERCA transgenic mice, pressure
overload hypertrophy is attenuated and prevented.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2002
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|