 |
|
|
 |
| Frontier of Atherosclerosis Research |
|
|
|
 |
|
Hematopoietic Stem Cells Differentiate into Vascular Cells that Participate in the Pathogenesis of Atherosclerosis
Masataka Sata
University of Tokyo Graduate School of Medicine, Tokyo, Japan
|
|
Circulating smooth
muscle progenitor cells may contribute to neointima
formation, hypothesized these
investigators. They first performed a heterotopic
cardiac transplantation between wild type and LacZ
mice that express
the marker gene LacZ in all tissues. Ninety percent
of the neointimal cells were LacZ-positive originating
from the recipient mice when a wild-type heart was
transplanted in a LacZ mouse, and conversely LacZ
negative cells for neointimal formation were observed
when the LacZ heart was transfected into the wild-type
mouse. The medial cells remained in the media. Recipient-derived
LacZ positive cells express various smooth muscle
markers, including myosin heavy chain, b-Caldesmon,
Calponin, and actin.
To investigate the
potential source of recipient cells that may contribute
to graft vasculopathy, they performed bone marrow
transplantation from GFP mice to wild-type mice. Wild-type
hearts transfected into GFP-BMT mice,
resulted in accumulation of bone marrow-derived cells
(BMDC), expressing smooth muscle actin, along graft
coronary arteries.
|
 |
|
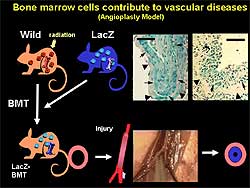
Figure
1. Angioplasty model used to study the contribution
of bone marrow cells to vascular remodeling.
|
| Click
to enlarge |
 |
|
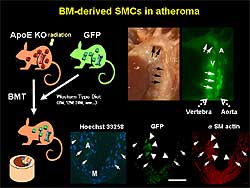
Figure
2. Model used to study the potential contribution of bone marrow cells
to atherosclerosis. Severe atherosclerosis is
shown in the center panel. The site of injection
is shown in the upper right panel. The cross-section
is shown in the lower right panel.
|
| Click
to enlarge |
|
The contribution of bone marrow cells to
vascular remodeling after mechanical injury was studied
by inserting a large wire into a femoral artery of
the LacZ-BMT mouse, leading to endothelial denudation
and marked enlargement of the lumen with rapid onset
of medial smooth muscle cell apoptosis. After 4 weeks,
the injured artery developed neointima hyperplasia;
60% of neointima and 40% of media were composed of
LacZ-positive cells originating from bone marrow (Figure
1).
A time course study
showed that in the absence of injury, no BMDC were
observed at the artery. At 1 week after injury, BMDC
accumulated on the injured artery. At 4 weeks, BMDC
contributed to neointima formation and re-endothelialization.
The potential contribution
of BMDC to atherosclerosis was then studied. Transplantation
was performed of BMDC from GFP mice to ApoE knockout
mice, which developed severe hyperlipidemia and atherosclerosis.
Injected cells settled at the bone marrow of the vertebra. When mice developed atherosclerosis, injected cells
also accumulated at the aorta, where severe atherosclerosis
developed. On cross-section, GFP-positive bone marrow
cells expressed smooth muscle actin. (Figure
2).
|
|
In LacZ to ApoE
knockout GFP-BMT, about 50% of smooth muscle actin
cells were derived from bone marrow. Media smooth
muscle cells were negative for LacZ, indicating a
specificity for this method. Anti-LacZ immunogold
labeling detected BMDC with muscle fiber, which may
be called smooth muscle-like cells. But in wild type
mice, they found medial smooth muscle cells with a
large body size and containing many secretory organelles.
These results suggest
that BMDC can give rise to vascular progenitor cells
that form as injured artery and differentiate and
proliferate and thus contribute to vascular remodeling
and neointima formation. However, another group reported
that BMDC do not contribute to neointima formation
in a model of aortic transplantation. Hence, the origin
of neointima cells is diverse.
Thus, this group
compared the contribution of BMDC using 3 different
models of neointimal hyperplasia. Inserting a large
wire to cause severe injury resulted in wire-mediated
expansion. Ligation of the common carotid artery resulted
in neointima containing a few BMDC. The placement
of a cuff around the right femoral artery induced
neointimal hyperplasia, after which many BMDC were
observed at the site of injury. However, most of the
cells were inflammatory cells in adventitia. Surprisingly,
no BMDC were found in the neointima.
To determine the
mechanism by which the contribution differs, they
investigated the vascular changes induced by wire,
cuff, and ligation injury. After wire injury, most
of the medial cells are killed by apoptosis. In contrast,
the cuff or ligation model induced mild injury and
smooth muscle medial cells remained relatively intact.
In the cuff model, inflammatory cells enter, smooth
muscle cells or other advential cells migrate and
proliferate and cause neointima formation. For a previously
severe injury, such as wire, most of the cells are
killed and BMDC are recruited to repair the vasculature.
The degree to which
BMDC contribute to the pathogenesis of human disease
is unknown. However, human pathology visualizes neovascalurization
and microhemmorhage in human atherosclerosis. Other
investigators have documented intima medial surface
damage and accumulation of fresh blood cells inside
the atheroma, suggesting those blood cells may contribute
to the remodeling of advanced atherosclerosis.
This group also
detected apoptosis of endothelial cells and smooth
muscle cells in advanced atherosclerosis. So they
investigated the contribution of BMDC to the progression
and remodeling of established atherosclerosis. They
replaced bone marrow using very old ApoE knockout
mice with advanced atherosclerosis. At 3 months, many
BMDC were seen in atheroma, mostly macrophages or
T cells. However, they also identified BMDC and luminal
endothelial cells, indicating those cells contributed
to vascular remodeling. Notably, they found the BMDC
also contributed to the neovascularization inside
the atheroma. BMDC were seen at the site of calcification,
which expressed osteoblast-related proteins. Thus,
they speculated that circulating progenitor cells
may play a role in the initiation and progression
of remodeling and the calcification of advanced atheroscleroisis.
BMDC may participate in cell turnover and repair after
apoptotic cell death.
|
 |
|
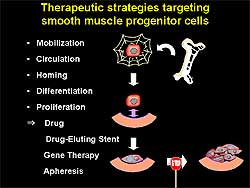
Figure
3. Potential therapeutic
strategies to target smooth muscle progenitor
cells.
|
| Click
to enlarge |
|
Consistent
with their results, another group reported that BMDC
are present in human coronary atherosclerosis. That
group analyzed patients with leukemia who received
gender mis-matched bone marrow. At 90 days, BMDC were
seen in coronary arteries. This phenomenon was more
prominent when the artery was diseased.
Sata and colleagues
are now developing therapeutic strategies targeting
smooth muscle progenitor cells (SMPC). Possible targets
include mobilization,
circulation, homing, differentiation and proliferation
of SMPC (Figure
3). The delivery
of a high concentration of a drug at the site of SMPC
accumulation may be beneficial.
|
PAGE
TOP
|
An Increase in Plasma Ox-LDL Levels during Coronary Artery Bypass Grafting are Associated with Plaque Injuries of the Aorta
Shoichi Ehara
Osaka City University Graduate School of Medicine, Osaka, Japan
|
|
Recently, oxidized
LDL (ox-LDL) is considered to play a key role in the
pathogenesis of the inflammatory process in atherosclerotic
lesions.
This group recently
developed a sensitive method to measure the plasma
levels of ox-LDL. Blood samples were collected in
a tube containing EDTA, and LDL fractions were obtained
from the samples by sequential ultracentrifugation
for 2 days. Standard ox-LDL measurement was prepared
by incubation of LDL from healthy volunteers with
CuSO4 at 37 degrees for 3 hours. Diluted
LDL fractions were added to microtiter wells precoated
with an anti-ox-LDL antibody (DLH3). After extensive
washing, the remaining ox-LDL was detected with a
sheep anti-human ApoB antibody and an alkaline phosphatase-conjugated
anti-sheep Ig G antibody. The measurement of plasma
levels of ox-LDL used a standard curve (ng/5 Äæg LDL
protein).
The characteristics
of the ox-LDL method developed by this group include
1) the use of LDL fraction from the blood plasma,
but does not use whole blood plasma, 2) minimizes
potential interference with other plasma constituents,
such as ox-VLDL, anti-oxidized LDL autoantibodies,
and anti-phospholipid antibodies, and 3) takes 4 days
to perform each measurement, but is superior to other
methods in obtaining a sensitive and accurate detection
of ox-LDL. A continuum of degrees of oxidation exists.
Oxidized phospholipids are a prominent component of
minimally modified LDL and fully ox-LDL.
|
 |
|
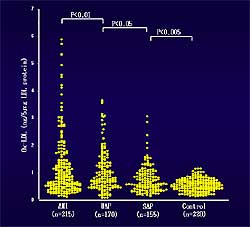
Figure
1. Oxidized LDL (ox-LDL) levels were significantly higher in patients
with acute myocardial infarction than in patients
with unstable angina, stable angina, or control
patients.
|
| Click
to enlarge |
|
This group previously
reported that elevated levels of ox-LDL show a positive
relationship with the severity of acute coronary syndromes
(ACS). Patients with acute myocardial infarction had
significantly higher levels of ox-LDL than in patients
with unstable angina, stable angina, or control patients
(Figure
1). Macrophage levels are also elevated. Their
findings suggest a pivotal role for ox-LDL in the
genesis of coronary plaque instability and the development
of ACS.
They investigated
the plasma levels of ox-LDL in patients who had undergone
coronary artery bypass grafting to determine whether
there is a relationship between plasma ox-LDL levels
and the release of aortic atheromatous debris due
to aortic clamp manipulation
in CABG patients.
|
|
Plasma ox-LDL levels
were measured in 3 groups: 1) CABG group of 27 patients
who had cardiopulmonary bypass and aortic clamp manipulation,
2) Off-pump CABG group of 6 patients, without aortic
clamp manipulation, and 3) non-CABG cardiac surgery
group of 6 patients who had atrial septal defect (ASD)
or mitral valve prolapse and aortic clamp manipulation
as a control group. In the non-CABG group, although
aortic clamp manipulation was performed, no atherosclerosis
of the ascending aorta was seen. Both the CABG and
Off-pump surgery group had mass aortic atherosclerosis.
Blood samples were
obtained on admission, and then post-operatively at
6 hours, day 1, day 3, and day 5. Epiaortic ultrasonography
of the ascending aorta was performed during surgery
to evaluate the severity of aortic atherosclerosis.
Based on the severity of atherosclerosis, the patients
were divided into 2 groups: mild atherosclerosis with
intimal thickness < 3 mm and severe atherosclerosis
with intimal thickness > 3 mm.
|
 |
|
Figure
2. The time course of ox-LDL levels in the study groups.
|
| Click
to enlarge |
 |
|
Figure
3. The increase in plasma ox-LDL levels followed by a decrease in the
severe atherosclerosis group suggests a balance
mechanism between oxidation and anti-oxidation.
|
| Click
to enlarge |
|
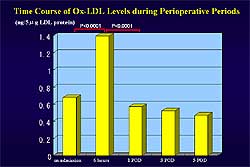
The time course
of ox-LDL levels during the peri-operative period
in the 3 groups showed in the CABG group a marked
and significant increase in plasma ox-LDL at 6 hours
and throughout until day 5 (Figure
2). In contrast, off-pump and non-CABG groups
did not have an increase in plasma ox-LDL levels.
Moreover, plasma ox-LDL levels at 6 hours were significantly
higher in the severe atherosclerosis group compared
to the mild atherosclerosis group. In the severe atherosclerosis
group, plasma ox-LDL levels showed a maximum increase
at 6 hours post-op and a subsequent decrease from
day 1 post-op onward. In contrast, in the mild atherosclerosis
group, no increase in the plasma ox-LDL levels was
seen.
These results support
the hypothesis that ox-LDL and atheromatous debris
present within severe atherosclerotic plaques of the
ascending aorta may be released into the blood stream,
most probably during aortic clamp manipulation during
CABG.
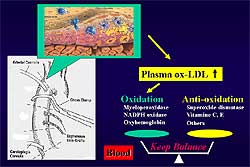
A balance mechanism
between oxidation and anti-oxidation in the blood
in humans is strongly suggested by the increase in
plasma ox-LDL levels at 6 hours post-op that subsequently
decrease in the severe atherosclerosis group (Figure
3). It has been suggested that anti-oxidants
would be able to inhibit cell-induced oxidative modifications.
Moreover, previous experimental studies have shown
that ox-LDL injected intravenously disappears from
plasma with a very short half-life, only minutes in
the rat or rabbit. These data strongly suggest that
a balance between oxidation and anti-oxidation may
play a role to determine plasma levels of ox-LDL in
humans.
|
PAGE
TOP
|
Graft Arterial Disease: A Model for Investigating Inflammation and T-cell Mediated Immunity in Coronary Atherosclerosis
Mitsuaki Isobe
Tokyo Medical and Dental University , Tokyo, Japan
|
|
Graft arterial disease
(GAD) is one of the most serious complications after
heart transplantation. Autopsy findings include nearly
total occlusion of coronary artery vessels. More than
50% of cardiac transplant recipients have some extent
of GAD at 5 years after transplantation.
Chronic rejection
cannot be prevented by conventional immunosuppressants
like tacrolimus or cyclosporin A. Therapeutic doses
of tacrolimus can prevent acute rejection but not
chronic rejection. A very high dose of tacrolimus
can reduce the extent of intimal hyperplasia to some
extent. But if the immunological tolerance is induced
to cardiac allograft the intimal hyperplasia or chronic
rejection is nearly completely prevented. This is
indirect evidence that T-cell mediated immunity is
involved in this processes.
The thickening intima
is comprised of smooth muscle cells (SMCs). The natural
form of SMCs decreases in the thickened intima, but
the synthetic form of immature SMCs is increased in
the thickened intima. This proliferation of SMCs can
be prevented to some extent by oral tranilast. The
decrease in SMCs proliferation is associated with
the induction of p21 expression,
which is a major cell cycle inhibitor genes.
Thus, this group
thought that T cells could activate SMCs for proliferation.
An in vitro study in which SMCs were taken
from mice aorta and co-cultured with T cells was performed.
SMCs were not activated by naïve T cells, but
activated T cells from mice with rejecting cardiac
allograft activated the SMCs proliferation. This proliferation
is associated with induction of PDGF, basic FGF, VCAM-1,
SMemb, and GAPDH. SMCs proliferation was prevented with antisense
Egr-1 transfected onto the SMCs prior to the co-culture,
suggesting that activated cells can interact with
SMCs and causes their proliferation.
T-cells require
two signals for optimal activation. One signal is
from the antigen via T-cell receptor, and the other
is a co-stimulatory signal for T-cell activation.
In addition to CD28, a few novel costimulatory molecules
have been identified, which are thought to play a role in the interaction of T-cells and SMCs. CD28 is
the major co-stimulatory molecule and studied for
more than 10 years. Fetal protein and immunoglobin
of CTLA4 binds to the ligand for CD28 and blocks its
signaling.
|
 |
|
Figure
1. Chronic rejection is nearly completely suppressed
by CTLA4-Ig.
|
| Click
to enlarge |
|
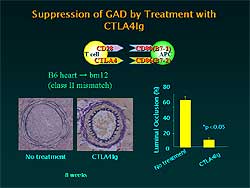
The induction of
CTLA4-Ig is a strong agent for inducing immunological
tolerance. But at the same time, chronic rejection
is nearly completely suppressed (Figure
1).
In the case of CD28,
because acute and chronic rejection is suppressed,
it is not possible to differentiate the mechanism
of chronic rejection from acute rejection. They found
that a novel molecule, iCOS, inducible co-stimulator,
a member of the CD28 family, was expressed on activated
T-cells and also SMCs. It appears that the iCOS expression
in the thickened intima plays some role in the interaction
between T cells and SMCs. If the iCOS pathway is blocked
by either anti-iCOS antibody or iCOS-Ig the development
of intimal hyperplasia is nearly completely blocked.
|
|
The same observation
was made with another co-stimulatory molecule, HVEM
(herpes virus entry mediator). The immunological role
of HVEM is not clearly understood. When mice were
treated with HVEM Ig, the intima hyperplasia is suppressed
in association with a reduction in the cytokines interferon
gamma, IL6, and IL4.
Another interesting
molecule is PD-1 (programmed death-1). PD-1 knockout
mice show dilated cardiomyopathy, probably due to
autoimmune mechanisms. PD-1 is believed to transduce
negative co-stimulatory signals to T-cells. PD-1 has
two ligands, PD-L1 and PD-L2. These investigators
used an PD-L1 antibody to block PD-L1 and found that
PD-L1 is expressed on thickened intima of recipient
mice. Blockade of PD-L1 signaling with the PD-L1 antibody
promotes the neointima formation compared to controls.
The same observation was made in a model of arterial
injury. After wire injury of mice femoral artery,
the neointima thickening was exaggerated with the
PD-L1 antibody.
There are many players
of inflammation, including ICAM-1, MCP-1, TNF-R, MMP,
and NF-κB. Adhesion molecules work as key players
of the inflammation by recruitment of white blood
cells to the site of inflammation. These investigators
used gene transfer technology to the donor heart.
When antisense ICAM-1 was transfected just prior to
transplantation, neointima formation was dramatically
reduced in association with reduction in cytokines.
MMP is a key molecule
required for tissue inflammation and tissue fibrosis.
MMP expression is induced on the thickened intima
of recipient mice in the chronic rejected heart. Also,
in the monkey model, tissue inhibitor of MMP was induced
in the outer area of the occluded vessel. These investigators
tried to block the expression of MMP-2 by using a
transfection of ribozymes to the heart allograft.
MMP-2 ribozyme nearly completely blocked the neointima
formation.
Cytokines also play
an important role in inflammation. TNF receptor knockout
mice (receptor 1, receptor 2, and double knockout)
were studied and showed that neointima hyperplasia
was blocked only when the double knockout mice were
transplanted into the wild-type recipient. The TNF
receptors on the donor heart are essential for the
development of neointima hyperplasia.
|
 |
|
Figure
2. The mechanism of action NF-κB, a key transcription
factor required for inflammation.
Figure
3. Hypothesis for the mechanism of coronary
graft arteriosclerosis.
|
| Click
to enlarge |
 |
|
Figure
3. Hypothesis for the mechanism of coronary
graft arteriosclerosis.
|
| Click
to enlarge |
|
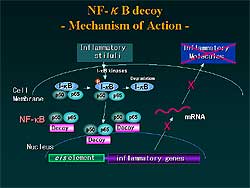
They tried to block
inflammation using NF-κB, a key transcription factor
required for inflammation (Figure
2 ). When synthetic DNA was transfected into the
cell, the DNA works as a decoy and blocks the production
of inflammatory molecules such as cell adhesion molecules
and cytokines. When the decoy is transfected into
the recipient mice heart just prior to transplantation,
neointimal formation is nearly completely blocked.
This reduction in chronic rejection is associated
with the reduction of the expression of VCAM-1 and
PDGF transcription.
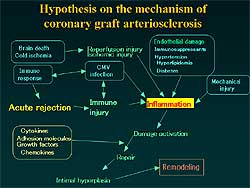
An hypothesis for
the mechanism of GAD developed by these investigators
based on their data and that from other investigators
and from clinical observations is shown in Figure
3.
Inflammation of
coronary arteries plays a key role. Inflammation is
caused by several factors: acute rejection causing
inflammation of the coronary arteries;
cytomegalovirus infection causing inflammation
directly or through acute rejection; ischemia and
reperfusion injury during the cold ischemia for preservation
of the donor heart; mechanical injury of the donor
heart during transport to the recipient hospital;
immunosuppressants, hypertension, hyperlipidemia,
and diabetes cause endothelial damage that promotes
inflammation. After the damage activation, the coronary
arteries show intimal hyperplasia and subsequent remodeling
of the coronary arteries.
GAD of the transplanted
heart and the restenosis after PTCA can occur on the
same molecular basis as inflammation. The stimulus
that causes GAD is immune injury to the whole coronary
arteries. Local mechanical injury is the stimulus
after PTCA.
In conclusion, T
cell-mediated immunity and inflammation are tightly
involved in the pathogenesis of GAD. Interventions
on T cell-mediated immunity through suppression of
costimulatory pathways and inhibition of inflammation
by gene therapy would be effective for the attenuation
or prevention of GAD.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2003
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|