 |
|
|
 |
| Restenosis after PCI 2003: New
Therapies for the Next Generation |
|
|
|
 |
|
Plaque
Composition Such as Lipid Core and Fibrous Cap Determine
Neointimal Formation Associated with In-Stent Restenosis:
Results from a Clinicopathologic Study
Hatsue Ishibashi-Ueda
National Cardiovascular Center,
Osaka, Japan
|
|
Atheromatous plaque composition, especially the presence
of a large lipid core associated with a thin fibrous
cap, is the most important element for excessive neointima
formation in the development of in-stent restenosis
(ISR), based on results from a study conducted by
this speaker. Thus, stabilizing plaques and reducing
the lipid core volume with statin therapy may inhibit
neointima proliferation and thereby prevent ISR.
Post-stent restenosis remains a serious problem.
Predicting the occurrence of ISR, associated with
extensive neo-intima formation, is important. Several
morphological determinants of neointima formation
have been proposed, including pre-stent atheromatous
plaque volume, plaque composition, positive remodeling
due to vessel wall over-stretching/ vascular injury,
thrombogenic factors and inflammation.
|
|
Study design
The present study by Ishibashi-Ueda and colleagues
sought to 1) clarify histologically the structure
of coronary arteries with in-stent restenosis compared
to those without restenosis, and 2) evaluate the relation
between native atheromatous plaque and neointimal
formation in stented coronary arteries.
|
|
|
They examined 34 stented segments from 25 autopsied
cadavers; the average age was 70 years, and 80% were
male. Forty percent were hyperlipidemic and 24% treated
with a statin. The stent implantation period was defined
as 30 days for their histologic analysis, and the
average was 198 days. The target vessel was LAD in
53% and right coronary artery in 32%. An acute myocardial
infarction (AMI) was the indication for stenting in
56% and unstable angina in 28%. Figure
1 outlines the clinical characteristics in the
study patients.
|
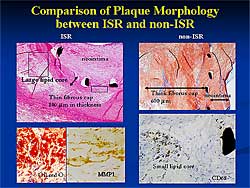 |
| Figure
2. A representative comparison of coronary artery
sections with and without in-stent restenosis.
|
| Click
to enlarge |
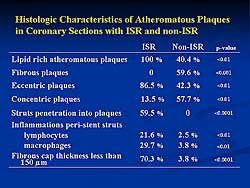 |
| Figure
3. The histological characteristics of the atheromatous
plaque in coronary sections with and without in-stent
restenosis. |
| Click
to enlarge |
|
A representative comparison of coronary artery sections
with and without ISR, on a micrograph, showed an atheromatous
plaque protruding outward representing remodeling,
with a large lipid core, occupying about 60% of the
atheromatous plaque (Figure
2). In the stented area, proliferating neointima
nearly occluded the lumen. In contrast, a concentric
fibrous plaque with no obvious lipid-rich core was
seen in the sections in non-ISR; the neointima was
thin and compact, and the lumen was largely open,
even 6 months post-stenting.
Under higher magnification, plaque morphology revealed
that in ISR the stent strut penetrated into the fibrous
cap and a large lipid core was under the stent strut.
Oil red O staining showed numerous lipid-laden macrophages.
In the non-ISR case, a thick fibrous cap was seen
beneath the stent and in a deeper layer a small lipid-rich
core composed of CD68 positive macrophages.
A detailed histology of peri-stent strut in ISR
showed that the stent implanted in a lipid-rich core,
neovascularization, lymphocyte infiltration, and foreign
body giant cells, and CD68 positive macrophage infiltration.
Figure
3 outlines the histological characteristics of
the atheromatous plaque in coronary sections with
and without ISR. In ISR, all the plaques were lipid-rich;
no fibrous plaque was present. The plaques were primarily
eccentric (86.5%), 13.5% concentric, and 59.5% of
struts penetrated into plaque. Peri-stent strut inflammation
was 21.6%s lymphocytes and 29.7% macrophages. In non-ISR,
plaques were lipid rich (40.4%) or fibrous (59.6%),
and were eccentric (42.3%) or concentric (57.7%);
no struts penetrated into plaques. Peri-stent strut
inflammation was limited.
Figure
4 outlines the morphometric results at the stented
sites. Although remodeling was one of the determinants
of ISR, their data showed no significant difference
in the remodeling index between ISR and non-ISR (1.26
vs 1.19, respectively; Cox hazard ratio (HR) 1.08;
p=0.39). However, the ratio of the lipid-rich core
to plaque area was much larger in ISR compared to
non-ISR (0.66 vs 0.34; Cox HR 2.48; p=0.011). The
thickness of the fibrous cap was thinner in ISR (140.4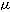 m
vs 317.8 m
vs 317.8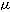 m
in non-ISR; Cox HR 3.5; p<0.0001). A larger ratio
for the neointima area to stent area was found in
ISR versus non-ISR (0.81 vs 0.29, respectively; Cox
HR 17.29; p=0.025). A positive correlation between
the amount of neointima and the lipid rich core burden
was found (r=0.79; p<0.001). m
in non-ISR; Cox HR 3.5; p<0.0001). A larger ratio
for the neointima area to stent area was found in
ISR versus non-ISR (0.81 vs 0.29, respectively; Cox
HR 17.29; p=0.025). A positive correlation between
the amount of neointima and the lipid rich core burden
was found (r=0.79; p<0.001).
|
PAGE
TOP
|
Pre-Procedural
Small Platelet Aggregates Predict Restenosis After
Percutaneous Coronary Intervention
Shinzo Miyamoto
Graduate School of Medical Sciences,
Kumamoto University, Kumamoto, Japan
|
|
Previous studies have shown that platelet aggregation
at follow-up is greater in patients with developing
restenosis. However, conventional assessment
of pre-procedural platelet aggregation has not provided
powerful predictors of restenosis after a percutaneous
intervention (PCI). A novel platelet aggregometer
using laser-light scattering has been shown to quantitatively
evaluate aggregate size and number.Small platelet
aggregates ultimately develop into medium and then
large platelet aggregates as platelet aggregation
proceeds. Because small platelet aggregates indicate
the first step in the development of platelet aggregation,
their measurement may be an important marker of initial
thrombus formation.
|
|
Study design
To elucidate the relation between platelet aggregation
and restenosis post-PCI, Miyamoto and colleagues evaluated
189 of the 196 enrolled patients (96% follow-up; 54
with acute coronary syndrome (ACS), 135 with stable
angina).
All patients had characteristic symptoms or subjective
clinical evidence of myocardial ischemia.The angioplasty
procedure was performed by the femoral approach according
to standard techniques.Angioplasty success was defined
as a <50% residual luminal stenosis of the dilated
segment using visual estimation, without major complications.
Coronary artery stenosis was assessed in two or more
projections before and after angioplasty and at follow-up.
All patients received standard medical therapy during
the follow-up period.
Follow-up coronary angiograms for asymptomatic patients
were performed 4 to 12 months after PCI.Restenosis
was defined as luminal reduction of ÿ50% at the previous
PCI site. Stenosis was assessed using quantitative
coronary angiography (QCA). All patients who had elective
PCI were treated with a low dose (100mg) of aspirin
daily and 200 mg of ticlopidine for 4 weeks when stents
were implanted. Written informed consent was obtained
from all patients.
In patients with stable angina, peripheral venous
blood was drawn after an overnight fast before elective
PCI.In patients with ACS, blood samplings were drawn
before initiating intravenous heparin injection and
before emergent PCI. Samples for platelet aggregation
were left for 15 minutes at room temperature, and
then centrifuged at 150 g for 10 minutes at room temperature
to obtain platelet-rich plasma. The other samples
were then centrifuged at 300 g for 10 minutes at room
temperature to obtain platelet-poor plasma.
Platelet aggregation was measured with a PA-200-instrument.
ADP 1.0  m
was used as an aggregating agent and added to platelet-rich
plasma 60 seconds after initiating the measurement.
Platelet aggregation was evaluated as the maximum
value of light intensity induced by ADP. m
was used as an aggregating agent and added to platelet-rich
plasma 60 seconds after initiating the measurement.
Platelet aggregation was evaluated as the maximum
value of light intensity induced by ADP.
|
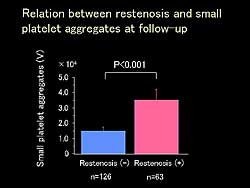 |
| Figure
2. The relation between restenosis and small platelet
aggregates at follow-up. |
| Click
to enlarge |
|
The clinical characteristics of the study patients
are outlined in Figure
1. Based on pre-procedural small platelet aggregation,
patients were divided into 3 percentiles: <50 percentile,
50-75 percentile, and >75 percentile. A significantly
higher triglyceride level was found in the >75
percentile (149 mg/dl vs 108 mg/dl in 50-75 percentile;
p<0.05). A greater percentage of patients in the
>75 percentile had ACS (51.7%) compared to 14.9%
in the <50 percentile and 41.7% in the 50-75 percentile
(p<0.01).
Restenosis rate was significantly higher in the
ACS group compared to the stable angina group (43.5%
vs 25.5%, p<0.05). In the 63 patients with restenosis,
small platelet aggregation was significantly greater
than in the 126 patients without restenosis (volume
of 3.4x104 vs 1.5x104 ; p<0.001;
Figure
2). Figure
3 shows the relation between restenosis and pre-procedural
small platelet aggregates.
The type of lesion and pre-procedural small platelet
aggregates were significant predictors of post-PCI
restenosis on multivariate logistic regression analysis.
However, only pre-procedural small platelet aggregates
was an independent predictor of post-PCI restenosis
(Figure
4). A preliminary study by this group shows that
ticlopidine, compared to aspirin, significantly decreased
the
|
| |
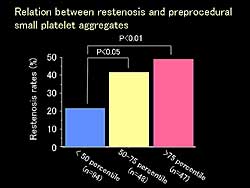 |
| Figure
3. The relation between restenosis and pre-procedural
small platelet aggregates. |
| Click
to enlarge |
|
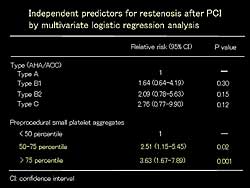 |
| Figure
4. Independent predictors for restenosis after
PCI by multivariate logistic regression analysis.
|
| Click
to enlarge |
|
|
Treatment recommendations
Based on their data, to prevent post-PCI restenosis
they recommend: In AMI patients, reperfusion should
be performed quickly in the acute phase, and in the
chronic phase, pretreatment before PCI for residual
stenosis. In patients with unstable angina, in the
acute phase use drugs for stabilization if urgent
coronary angiogram and PCI are not needed. In the
chronic phase, pretreatment before PCI. In patients
with stable angina, pretreatment before PCI.
|
PAGE
TOP
|
Potent
Inhibitory Effects of Sirolimus on Circulating Smooth
Muscle Progenitor Cells
Daiju Fukuda
University of Tokyo, Tokyo, Japan
|
|
Sirolimus-eluting stents have emerged as a promising
strategy to prevent in-stent restenosis (ISR). Sirolimus
is a macrolide antibiotic with potent antifungal,
immunosuppressive. and antimitotic properties. Sirolimus
binds to a specific cytosolic protein, FK506 binding
protein 12 (FKBP). The sirolimus FKBP complex binds
to a specific cell cycle-regulatory protein, the mammalian
target of rapamycin (mTOR), and inhibits its activation.
The inhibition of mTOR ultimately induces cell-cycle
arrest in the G1 phase and consequently arrests cell
growth. Restenosis rates and clinical events in patients
with complex lesions have been reduced in randomized
clinical trials of sirolimus-eluting stents
The inhibitory effect of sirolimus on smooth muscle
cell (SMC) proliferation and migration in vitro has
been demonstrated. However, the exact mechanism by
which locally delivered sirolimus prevents ISR is
unknown. Although neointimal hyperplasia resulting
from the excessive accumulation of SMC is the primary
cause of ISR, the origin of neointimal SMC is not
well understood.
This group recently demonstrated that bone marrow
cells give rise to smooth muscle progenitor cells
(SMPCs) that accumulate at the site injured arteries,
differentiate, and contribute to neointimal hyperplasia.
Neointimal SMCs are thought to derive from the media
and from circulating SMPCs.
|
|
Study design
The effect of sirolimus on SMPCs was therefore studied
by this group. In their assay system for SMPCs, peripheral
mononuclear cells were isolated from the blood of
healthy human volunteers by density gradient centrifugation.
The isolated mononuclear cells were then cultured
in fibronectin-coated wells with basic FGF and PDGF-BB.
At day 14, the cells were differentiated into alpha-smooth
muscle actin positive cells, which were then defined
as smooth muscle-like cells. The assay was performed
in the presence of sirolimus, and actin was expressed
at both the mRNA and protein levels.
|
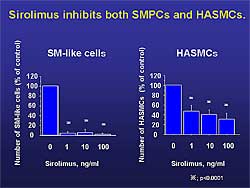 |
| Figure
1. Sirolimus inhibited the proliferation of human
aortic smooth muscle cells and differentiation
of SMPCs. |
| Click
to enlarge |
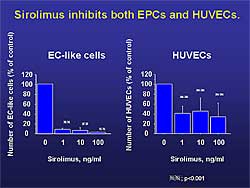 |
| Figure
2. Sirolimus mildly inhibited the proliferation
of human umbilical vein endothelial cells and
inhibited the differentiation of EPCs. |
| Click
to enlarge |
|
Mononuclear cells were cultured in the presence of
0 to 100 ng/mL sirolimus, which had little effect
at day 4 on the number of adherent cells. However,
at day 14, a dramatic decrease in the number of smooth
muscle-like cells by 1.0 ng/mL sirolimus was found
(2.4 vs 60.9 cells, p<0.001). Culturing human aortic
smooth muscle cells with sirolimus inhibited their
proliferation and the differentiation of SMPCs (Figure
1). However, SMPC differentiation was inhibited
more effectively than the proliferation of the human
aortic SMCs.
The expression of FKBP12 was more abundant in mononuclear
cells than in SMCs. Sirolimus is thought to have other
pathways, however this difference may be one explanation
for the present results, that is, sirolimus may target
cell types other than medial SMCs.
To investigate the effect of sirolimus on EPCs,
mononuclear cells were cultured with hydrocortisone
(1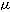 g/mL),
bovine brain extract (3 g/mL),
bovine brain extract (3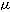 g/mL),
and VEGF (10 ng/mL). Endothelial-like cells
were defined as cells double positive for Dil-acetylated
LDL and FITC-lectin, as described in the literature.
Differentiation of mononuclear cells to endothelial-like
cells was inhibited, in the presence of sirolimus,
as were SMPCs. g/mL),
and VEGF (10 ng/mL). Endothelial-like cells
were defined as cells double positive for Dil-acetylated
LDL and FITC-lectin, as described in the literature.
Differentiation of mononuclear cells to endothelial-like
cells was inhibited, in the presence of sirolimus,
as were SMPCs.
Culturing with sirolimus mildly inhibited the proliferation
of human umbilical vein endothelial cells by 33.3%
and inhibited the differentiation of EPCs (Figure
2). These results suggest that sirolimus also
exerts an inhibitory effect on EPCs originating from
mononuclear cells, thereby affecting re-endothelialization.
|
|
Summary
Sirolimus has been reported to inhibit medial SMC
cell migration and proliferation.The present study
suggests that sirolimus inhibits the differentiation
of bone marrow-derived SMPCs to neointimal smooth
muscle cells. Further, these results suggest that
sirolimus also exerted an inhibitory effect on EPCs,
thus affecting re-endothelialization.
Potent inhibitory effects of sirolimus on circulating
SMPCs may, at least in part, mediate the clinical
efficacy of sirolimus-eluting stent. Sirolimus potently
affects re-endothelialization after stent-implantation.
|
PAGE
TOP
|
Effects
of Local Delivery of Evans Blue and Phenolsulfonphtaleine
on Migration of Vascular Progenitor Cells into the
Coronary Arterial Wall
Yasumi Uchida
Jikei University School of Medicine
Tokyo, Japan
|
|
Previous work by this group found that vascular progenitor
cells (VPCs) play an important role in restenosis
by migrating into coronary wall through three routes.
The aim of the present studies was to examine the
roles of VPCs in coronary restenosis and to develop
new treatment modalities by controlling migration
of VPCs.
|
|
Study design
In anesthesized beagle dogs, coronary arteries were
dilated by either balloon inflation (POBA) or by stent
implantation and the role of VPCs examined. Mononuclear
cells with beta-SM actin were considered ad VPCs because
they also had CD34 and other vascular markers. Following
mechanical intervention, VPCs migrated into the interstitial
space. VPCs were found to migrate into the intima
via 3 routes, from the adventitia, the lumen, and
from new vessels in hyperplastic intima. Direct migration
from the lumen into the intima was also observed.
The migration continues for more than 4 weeks.
To prevent migration of VPCs into the intima, treatment
approaches should include substances that prevent
migration of VPCs and of smooth muscle cells (SMCs)
pre-existing in the media. The substances should easily
diffuse into the entire wall, including the adventitia,
and remain there for more than 4 weeks. Stents should
elute drugs for more than 4 weeks.
Further experiments by this group showed that Evans
Blue (EB, 25 mg) or phenolsulfonphthaleine (PSP, 25
mg), delivered using a porous balloon into the dilated
middle to distal coronary segments of anesthetized
beagle dogs, prevented migration of VPCs, as found
on coronary angiography. POBA-induced intimal hyperplasia
was suppressed by EB and PSP. Also, POBA-induced stenosis
was suppressed by EB and PSP. A significant reduction
in the number of VPCs per unit area by the dyes was
found. At 6 months, multi-layered dye-eluting stents
(EB 20-25 mg) effectively prevented intimal hyperplasia
and stenosis. VPC migration was also suppressed.
To clarify the mechanisms for the beneficial effects
of these dyes, they compared the pharmacologic actions
of EB and PSP in vitro and in vivo. EB has inhibitory
actions on SMC proliferation, thrombosis, migration,
and stenosis, and inhibits the cell cycle at G1 and
inhibits von Willebrand factor action. In contrast,
PSP inhibits only migration and stenosis.
|
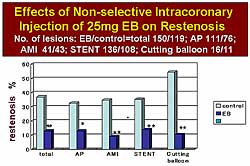 |
| Figure
1. An intracoronary bolus injection of Evans Blue
reduced restenosis in angina, myocardial infarction,
stent, and cutting balloon. |
| Click
to enlarge |
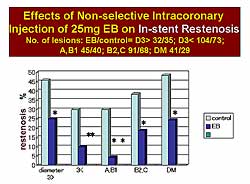 |
| Figure
2. Evans Blue reduced the in-stent restenosis
rate, regardless of the angiographic coronary
anatomy. |
| Click
to enlarge |
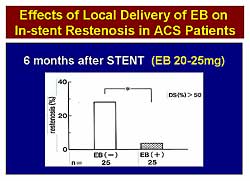 |
| Figure
3. Locally-delivered Evans Blue to the stented
segments reduced in-stent restenosis below 5%
in patients with myocardial infarction. |
| Click
to enlarge |
|
Based on the results of the experimental studies,
they conducted 2 open clinical trials using EB, which
had been clinically used for the measurement of cardiac
out put in patients. In Trial 1, EB (25 mg) was injected
into the treated arteries through a guiding catheter
immediately after PCI. In Trial 2, EB (20-25 mg) was
delivered locally by a porous balloon after PCI in
25 patients with ACS.
In Trial 1, the intracoronary bolus injection of
EB was associated with a reduction in restenosis in
the setting of angina, AMI, stent, and cutting balloon
(Figure
1). The restenosis rate was about 10% in the treated
groups. EB also reduced the in-stent restenosis (ISR)
rate, regardless of the angiographic coronary anatomy
(Figure
2). The ISR rate was below 5% in the type AB1
group. In Trial 2, local delivery of EB to the stented
segments also reduced the ISR rate below 5% in patients
with AMI (Figure
3).
|
|
Conclusions
Following PCI, VPCs migrate into the intima via 3
routes, through adventitia through the media, directly
from the lumen, and from neovasculature in the intima.
VPCs play an important role in coronary stenosis in
the canine model. The local delivery of EB and PSP
and the novel dye-eluting stents prevented the migration
of VPCs and inhibited PCI-induced coronary stenosis
in dogs. EB prevented PCI-induced restenosis in patients
with coronary artery disease. In addition to the inhibition
of thrombosis and migration of SMCs pre-existing in
the media, by preventing migration of VPCs into the
intima, complete restenosis prevention may be attained.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2004
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|