 |
|
|
 |
| Atrial Fibrillation: From Bench To Bedside |
|
|
|
 |
|
Structural
Remodeling in Atrial Fibrillation as Arrhythmogenic
Substrates: Tachyarrhythmia and Gap Junction Remodeling
Tomoko Ohkusa
Yamaguchi University School of
Medicine, Ube-Yamaguchi, Japan
|
|
Fibrosis, extracellular matrix, fiber orientation,
autonomics, Gap junction, ion channels, and calcium
homeostasis are known to be arrythmogenic substrates.
Remodeling is thought to trigger arrhythmias and facilitate
their occurrence, which can induce further remodeling.
The downregulation of the ryanodine receptor or Ca2+
ATPase and the upregulation of IP3 receptors might
cause an abnormal intracellular calcium homeostasis,
resulting in the initiation and perpetuation of atrial
fibrillation (AF).
|
|
|
Electrical coupling in the heart is mediated by Gap
junctions, specialized membrane lesions comprising
groups of channels directly connecting the cytoplasmic
compartments of two adjacent cells. Gap junction facilitates
the intercellular exchange of ions, signaling molecules,
and other molecular information, and affects impulse
propagation and/or electrical coupling (Figure
1). In the heart, at least 4 different connexin
(Cx) proteins are expressed, with short half-lives
of 1-2 hours. Phosphorylation regulates the stability
of the connection.
|
|
Chronic fibrillating human atrial myocardium
This group analyzed the expression of Cx40 and Cx43
in the right atrium of patients with mitral valvular
disease (MVD) with AF or in normal sinus rhythm (NSR)
and in controls. Cx40 mRNA expression was significantly
lower in MVD/AF patients, while no significant difference
was seen in for Cx43 between the 3 groups. Expression
of Cx40 protein was significantly lower in AF patients,
while for Cx43 protein there was no significant change.
Serine-phosphorylation, but not tyrosine phosphorylation,
was found for both Cx40 and Cx43 on tissue immunoprecipitation.
Notably, serine-phosphorylated Cx43 was about 50%
greater in patients with AF.
|
|
Cardiomyocytes exposed to rapid electrical
stimulation (RES) of contraction
|
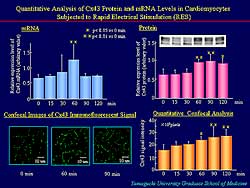 |
| Figure
2. In rat cultured ventricular myocytes, Cx43
mRNA was increased, Cx43 immunoreactive signals
without affecting distribution pattern, and Cx43
area was increased. |
| Click
to enlarge |
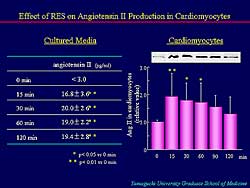 |
| Figure
3. RES markedly increased angiotensin II content
in cultured cardiomyocytes. |
| Click
to enlarge |
|
Cx43 mRNA was increased in rat cultured ventricular
myocytes, peaking at 90 minutes. Cx43 immunoreactive
signals were appreciably increased with no affect
on the distribution pattern (Figure
2). Quantitative confocal analysis revealed a
significant increase in the Cx43 area.
RES was associated with a marked increase in angiotensin
II content in the cultured cardiomyocytes (Figure
3). Angiotensin II is known to increase Cx43 expression.
However, in presence of losartan, RES did not significantly
increase Cx43 protein or Cx43 mRNA.
Nearly uniform propagation of excitation from left
to right was seen on multielectrode extracellular
potential mapping. In the cultured cardiomyocytes,
compared to controls, the activation times at the
recording sites after RES were much shorter—indicating
a RES-induced increase of propagation, which did not
occur in cardiomyocytes treated with losartan. In
the absence of losartan, RES increased the conduction
velocity after 30 minutes, while no significant changes
in conduction velocity by RES were seen in the presence
of losartan.
|
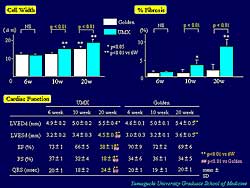 |
| Figure
4. In cadiomyopathic hamsters compared to age-matched
controls, after 20 weeks, left ventricular end
diastolic diameter significantly increased, ejection
fraction and fractional shortening decreased,
and QRS was significantly prolonged. |
| Click
to enlarge |
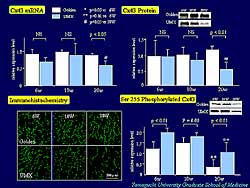 |
| Figure
5. In cardiomyopathic hamsters, Cx43 mRNA and
Cx43 protein decreased at 20 weeks, and Cx43 expression
on immunohistochemistry gradually decreased. Although
serine 255 phosphorylation of Cx43 gradually decreased
with age in both groups, it was significantly
greater in the cardiomyopathic hamsters compared
to the golden hamsters at 6 weeks and 20 weeks.
|
| Click
to enlarge |
|
In hamster cardiomyopathic hearts, cell width and
percentage of fibrosis gradually increased after 10
weeks. After 20 weeks, left ventricular end diastolic
diameter (LVEDD) significantly increased, and the
ejection fraction (EF) and fractional shortening decreased
in the cardiomyopathic hamsters compared to age-matched
control hamsters. QRS width was significantly prolonged
at 20 weeks (Figure
4). Quantitative analysis of Cx43 revealed that
the amount of Cx43 mRNA and Cx43 protein were decreased
at 20 weeks. In the cardiomyopathic hamsters, Cx43
expression on immunohistochemistry was gradually decreased
compared to golden hamsters (Figure
5). Quantitative analysis of serine 255 phosphorylation
of Cx43 showed its expression was gradually decreased
with age in cardiomyopathic and control hamsters.
Interestingly, in the cardiomyopathic hamster, the
expression of serine phosphorylated Cx43 was significantly
greater than that of golden hamsters at 6 weeks and
20 weeks.
|
|
Summary
In chronically fibrillating human atrial myocardium,
Cx40 was downregulated and abnormally phosphorylated,
but no changes were seen for Cx43.
In ventricular cardiomyocytes exposed to rapid electrical
stimulation of contraction, Cx43 was upregulated through
an autocrine action of angiotensin II to active MAP
kinases, resulting in potentially arrhythmogenic alteration
of conduction properties.
In cardiomyopathic hamster hearts, QRS interval was
prolonged with an increase of interstitial fibrosis
and downregulation and abnormal phosphorylation of
Cx43.
|
|
Conclusion
In addition to functional remodeling of cardiomyocytes,
gap junction remodeling might also cause abnormal
impulse propagation and electrical coupling, resulting
in the initiation and/or perpetuation of arrhythmias.
For new therapeutic target, the upstream approach
to regulating substrates involved in remodeling at
the molecular, cellular and/or organ level may be
a promising new approach in the treatment of tachyarrhythmias,
including AF.
|
PAGE
TOP
|
Effects
of Antiarrhythmic Drugs on Electrophysiological Action
and Wavefront Dynamics During Atrial Fibrillation
Takanori Ikeda
Kyorin University School of Medicine,
Mitaka, Japan
|
|
The wavelength theory, which states that the prolongation
of the wavelength (WL), either of the refractory period
(RP) or increment of conduction velocity (CV), has
been used to explain the efficacy of antiarrhythmic
drugs to terminate AF. However, the WL theory does
not fully explain the mechanism of action whereby
all antiarrhythmic drugs terminate AF.
Recently, the importance of the “temporal”
and “spatial” excitable gap (EG) for cardioversion
of AF has been proposed by Wijffels and colleagues.
The temporal EG, which is thought to be more important,
is calculated as the mean AF cycle length minus the
refractory period. The spatial EG is calculated as
the pathlength minus the wavelength. They state that
the temporal and spatial EG are widened, but the WL
not prolonged, just before cardioversion of AF with
multi-channel blockers, such as cibenzoline and flecainide.
The single meandering hypothesis and the mother
rotor hypothesis have shown that wavefront propagation
is complex, but an essential reentry exists during
AF. It has also been shown that the property of the
core is important for the maintenance of functional
reentry in the atrium, and the reentry terminates
when the core is excited by an outside wavefront.
|
|
Study design
To determine alternative mechanisms by which antiarrhythmic
drugs terminate AF, they hypothesized that a widening
of the EG, the enlargement of the core, and the presence
of outside wavefronts participate in cardioversion
of AF by antiarrhythmic drugs.
To test their hypothesis they studied pilsicainide
(PIL), a pure sodium channel blocker, whose high efficacy
cannot be understood because its main action is the
decrement of conduction velocity, and nifekalant (NIF),
a pure Ikr channel blocker, whose lack of efficacy
in AF is not understood, despite its ability to prolong
the WL and RP and its efficacy in ventricular fibrillation.
|
|
|
Mapping techniques were used to assess the effects
of PIL and NIF on electrophysiologic actions, including
the EG, and wavefront dynamics during AF. In a newly
developed model of isolated, perfused and superfused
canine atria, AF was induced with 1-5 mM acetylcholine
(Figure
1).
The right and left endocardia were simultaneously
mapped using a computerized mapping system.
PIL (2.5 mg/mL) or NIF (5 mg/mL) was perfused
after stabilization of induced AF.
Electrophysiological parameters and the core of mother
reentry (an essential reentry) were measured during
AF. An electrode array with 224 bipolar electrodes
was used. The temporal EG was calculated as the mean
AF cycle length (ms) minus the RP (ms).
|
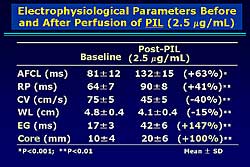 |
| Figure
2. The changes in electrophysiologic parameters
after pilsicainide administration compared to
baseline. |
| Click
to enlarge |
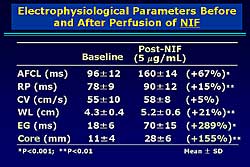 |
| Figure
4. The electrophysiologic parameters before and
after administration of nifekalant. |
| Click
to enlarge |
|
After perfusion of PIL, the AF cycle was increased
63%, the RP increased 41%, the CV velocity decreased
40%, and the WL decreased 15% (Figure
2). A significant increase in the EG and core
area were found (147% and 100%, respectively). In
8 of 12 preparations, AF was eventually terminated
with a cumulative PIL dose of 7.5 É g/mL.
In these 8 preparations, the activation pattern was
unorganized, despite the decrease in the number of
WL (Figure
3). AF was converted to an organized pattern in
the remaining 4 preparations.
With NIF, the activity was regular and uniform on
the bipolar electrograms. The reentrant wavefront
rotated in a counter clockwise direction. After NIF
perfusion, only a single reentrant wavefront can be
seen. The mean cycle length was increased 67%, the
RP increased 15%, the CV 21%, and the WL 21% (Figure
4). A marked widening of the EG and enlargement
of the core perimeter were found (289% and 155%, respectively).
Although NIF widened the EG and the core perimeter,
no outside wavefronts were observed. Further experiments
showed that with NIF organized AF was easily terminated
by a single pacing from the EG area. Thus, an outside
wavefront appears necessary to terminate AF.
|
|
Conclusions
Outside wavefronts, widening of the EG, and enlargement
of the core of the mother reentry are important for
cardioversion of AF by pure Na channel and Ikr channel
blockers, according to the data from the present study.
|
PAGE
TOP
|
Long-Term
Prognosis of the Patients with Paroxysmal Atrial Fibrillation:
Differential Responses to Antiarrhythmic Therapy
Takashi Komatsu
Iwai Hospital, Ichinoseki, Japan
|
|
A retrospective analysis of patients with paroxysmal
atrial fibrillation (AF) by Komatsu and colleagues
showed a variable long-term prognosis related to the
response to the antiarrhythmic therapy. Maintenance
of sinus rhythm (SR) was associated with a good prognosis,
even in the absence of anticoagulation therapy. AF
recurrence or development of permanent AF was associated
with a poor prognosis, with the possibility of stroke
in the absence of anticoagulation therapy. Personalized
selection of antiarrhythmic drugs is needed to improve
its efficacy. The development of a more effective,
more atrium-specific and safer drug is anticipated.
|
 |
| Figure
1. The protocol for antiarrhythmic drug therapy
in the present study. |
| Click
to enlarge |
|
To answer questions remaining after the AFFIRM and
RACE trials, these investigators sought to clarify
the role of antiarrhythmic drug therapy in the management
of patients with symptomatic paroxysmal AF and with
normal or minimally impaired left ventricular (LV)
function. They examined the 1) long-term efficacy
of antiarrhythmic drug therapy with class I drugs
and amiodarone, and 2) relationship between the efficacy
of antiarrhythmic drug therapy and the long-term prognosis
of the patients.
A total of 290 patients with symptomatic paroxysmal
AF (191 men, mean age 69 years) and an LV ejection
fraction (EF) > 40%, who were treated þ 1 a month
at the outpatient clinic for a minimum of 12 months
(mean follow-up 51 months) was included in the analysis.
According to the protocol for antiarrhythmic therapy,
after restoration of sinus rhythm (SR) a class 1 drug
was given by a sealed envelop method. For AF recurrence,
either flecainide, pilsicainide, or bepridil was given
by a sealed envelope method. If AF persisted, the
physician could freely select amiodarone or a different
class I drug. Figure
1 illustrates the protocol for drug therapy in
this study.
|
|
Study results
The efficacy of the antiarrhythmic drug in preventing
the recurrence of paroxysmal AF is limited. At 20
months after the first antiarrhythmic drug was given,
only 51% of the patients on disopyramide, 47% oncibenzoline,
and 35% on aprindine were free of AF recurrence. At
20 months after the second antiarrhythmic drug, only
33% of patients treated with flecainide and with pilsicainide
and 21% with bepridil remained free of AF recurrence.
After the addition of the third antiarrhythmic drug,
at 20 months, 44% of patients treated with amiodarone
and 60% with a class I antiarrhythmic drug remained
free of AF recurrence.
|
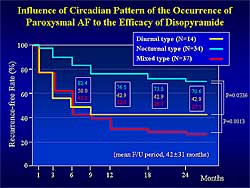 |
| Figure
2. Influence of Circadian pattern on the occurrence
of paroxysmal AF to the efficacy of disopyramide |
| Click
to enlarge |
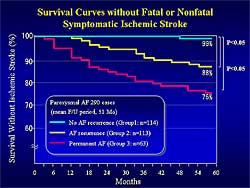 |
| Figure
3. Survival curves in patients without an ischemic
stroke. |
| Click
to enlarge |
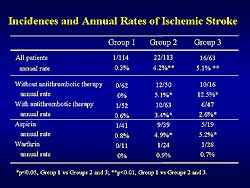 |
| Figure
4. The incidence and annual rate of ischemic stroke
in the study patients. |
| Click
to enlarge |
|
Selection of the antiarrhythmic drug based on the
clinical profile of the patient may improve efficacy.
A circadian variation has been shown in the paroxysmal
AF in many patients. The investigators divided the
patients taking disopyramide into 3 groups based on
the time of AF recurrence: diurnal (n=14), nocturnal
(n=34), and mixed (n=37). At 24 months after the initiation
of disopyramide, 71% of the nocturnal group was recurrence-free,
compared to only 43% of the diurnal group and 27%
of the mixed group (Figure
2). Therefore, disopyramide can be first-line
treatment for patients with nocturnal type of paroxysmal
AF without contraindications.
Based on the results of the antiarrhythmic therapy,
the patients were divided into 3 groups: SR without
recurrence (Group 1, n=114), AF recurrence during
therapy (Group 2, n=113), and conversion to permanent
AF (Group 3, n=63). The efficacy rates of 39%, 39%,
and 22%, respectively, are consistent with other reports,
showing the limited efficacy of antiarrhythmic drugs
to maintain SR.
At 16 months, no significant difference in survival
free of cardiovascular death was found between the
3 groups (99%, 95%, and 94%, respectively). Survival
free of fatal or nonfatal symptomatic ischemic stroke
was significantly higher in Group 1 compared to Groups
2 and 3 (99%, 88%, and 76%; p<0.05; Figure
3). Ischemic stroke occurred in only 1 patient
in Group 1. The incidence of ischemic stroke in patients
with AF clearly depends on the antithrombotic therapy
(Figure
4). At baseline, no significant difference was
noted in the prevalence of the risk factors for ischemic
stroke among the 3 groups. The total annual stroke
rate was lower in Group 1 than in Groups 2 and 3.
In patients in whom any antithrombotic therapy was
not given during follow-up, the annual stroke rate
was lower in Group 1 than in Groups 2 and 3. In patients
in whom antithrombotic therapy was given the annual
stroke rate was higher in Group 1 than in Groups 2
and 3. In patients taking aspirin, the annual stroke
rate was higher in Group 1 than in Groups 2 and 3,
while in the patients taking warfarin the annual stroke
rate was similarly low in each group.
|
PAGE
TOP
|
Map-Guided
Surgery for Atrial Fibrillation
Takashi Nitta
Nippon Graduate School of Medicine,
Tokyo, Japan
|
|
The Maze procedure cures atrial fibrillation (AF)
by isolating all four pulmonary veins (PVs) and by
the interrupting all possible reentrant circuits.
Although the success rate of the Maze procedure is
90%, it is a technically demanding procedure, involving
complex and extensive atrial incisions, bleeding risk,
40 to 50 minutes of cardiac arrest time often required.
Recently, isolation of the PV alone has successfully
cured AF in selected patients. The PV isolation procedure
is simple, safe, and can be completed within 10 to
15 minutes. However, the success rate of 50-70% for
permanent AF is significantly lower than with the
Maze procedure. Electrophysiologic-based surgery that
is less invasive needs to be established to address
some of the problems remaining with the current procedures.
|
|
Study design
To address this need, this group performed intra-operative
mapping in 45 patients (25 male). The average age
was 62 years, 82% had valvular heart disease, 4 patients
had congenital heart disease, and 4 patients no structural
heart disease. Paroxysmal AF was found in 9 patients
and 36 had permanent AF.
Custom-made electrodes were used for the intraoperative
mapping. An atrial mold from a cadaver heart was made,
and then the curvature of the atrial epicardium was
copied to 3 silicon patches, one each for the lateral
right atrium, the Bachmann’s bundle, and the
lateral left atrium. A total of 253 bipolar electrodes
were distributed over the 3 silicon patches. Nearly
the entire epicardium of the atria, including the
posterior left atrium between the pulmonary veins,
was mapped with the electrodes.
AF of 500-1000 ms duration was analyzed in each
patient. Activation sequence maps were displayed on
a 3-D atrial model as a movie. At least 10 minutes
were required for the analysis and more time was required
in complex activations.
|
|
Findings from intra-operative mapping
A representative map from a 72-year-old male patient
with mitral valve regurgitation, coronary artery disease
(CAD), and paroxysmal AF revealed at least 2 foci
of repetitive activation—one in the left superior
PV (LSPV) and the other is in the right superior PV
(RSPV). Propagation was toward the right atrium and
over the Bachmann’s bundle, because of the faster
activation from the LSPV than from the RSPV. The right
atrium activation was passive in this patient, and
he was cured of AF by PV isolation alone, without
incisions on the right atrium, because there was no
reentrant activation in the RA.
In a patient with permanent AF and mitral valve
disease, focal activation originated from the LSPV
and propagated toward the right atrium and over the
Bachmann’s bundle. Another focal activation originated
from the RSPV simultaneously. Left to right inter-atrial
conduction via Bachmann’s bundle demonstrated
so-called fibrillatory conduction. The electrograms
revealed a progressive conduction delay in the pathway
from the focus at the LSPV to the right atrium, resulting
in a variable conduction ratio and non-activated regions.
All patients had 2 to 4 foci in the left atrium,
mostly arising from the posterior left atrium adjacent
to the PVs, particularly the LSPV and RSPV. Interestingly,
this distribution is similar to that in paroxysmal
AF shown by Haissguerre and colleagues.
|
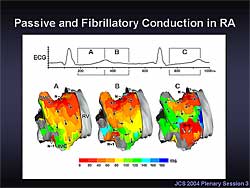 |
| Figure
1. In the passive activation in the lateral right
atrium during AF, the focal activation arose from
the pulmonary vein, conducted over the Bachmann’s
bundle, and appeared at the top of the right atrium.
A progressive conduction delay was seen in the
lateral right atrium, resulting in residual activation
from the preceding cycle and coexisting multiple
wavelets in the lateral right atrium. |
| Click
to enlarge |
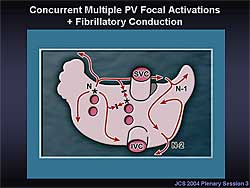 |
| Figure
2. In the basic pattern of atrial activation during
AF, concurrent multiple PV focal activations and
fibrillatory conduction are the dominant mechanism
in AF. Extremely rapid activations in the PV and
a progressive conduction delay or block in the
pathway from the focus to the right atrium and
in the lateral right atrium causes an irregular
and complex right atrial activation, resulting
in coexisting multiple wavelets. |
| Click
to enlarge |
|
In the passive activation on the lateral right atrium
during AF, the focal activation arose from the PV,
conducted over the Bachmann’s bundle, and appeared
at the top of the right atrium (Figure
1). The activation in the right atrium was passive,
with progressive conduction delay in the lateral right
atrium. The delayed conduction resulted in residual
activation from the preceding cycle and coexisting
multiple wavelets in the lateral right atrium. As
a result, the activation in the right atrium desynchronized
with the left atrial activation, and became irregular
and complex.
Concurrent multiple PV focal activations combined
with fibrillatory conduction is the dominant mechanism
in the basic pattern of atrial activation during AF
(Figure
2). Extremely rapid activations in the PV and
a progressive conduction delay or block in the pathway
from the focus to the right atrium and in the lateral
right atrium cause an irregular and complex right
atrium activation, resulted in coexisting multiple
wavelets.
The map from a 68-year-old male patient with mitral
valve stenosis, tricuspid valve regurgitation, and
chronic AF revealed focal activation arising from
the LSPV, and a reentrant activation around the right
atrial appendage (Figure
3). The patient required the radial procedure
comprising biatrial incisions in addition to PV isolation.
More than 50% of the study patients with permanent
AF had right atrium activation, but none of the paroxysmal
AF patients.
In 3 patients, the electrograms were low voltage
over the broad area in the left atrium. There was
a focal activation arising from the LSPV, and the
right atrium was activated by the activation conducted
from the left atrium (Figure
4). Surgery for AF was not indicated, because
of the low chance of converting AF to sinus rhythm,
and the poor atrial contraction even if sinus rhythm
resumed.
|
| |
|
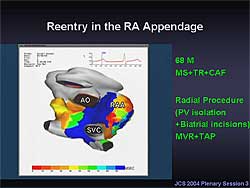 |
| Figure
3. Mapping in a 68-year-old male patient with
mitral valve stenosis, tricuspid valve regurgitation,
and chronic AF, showed focal activation arising
from the left superior pulmonary vein, and a reentrant
activation around the right atrial appendage.
|
| Click
to enlarge |
|
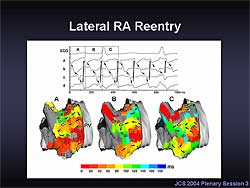 |
| Figure
4. In this example of right atrial reentry, 2
reentrant circuits were seen, one in the lateral
right atrium and one in the right atrial appendage.
The location and shape of the reentrant circuits
changed in each cycle. |
| Click
to enlarge |
|
|
Strategy for intra-operative mapping guided
surgery
Treatment approaches based on the findings from
intraoperative mapping are as follows. For PV focal
activations and passive conduction in the right atrium,
cure may be achieved by PV isolation alone. For right
atrial reentrant activations, the Maze or radial procedure
should be used to interrupt the reentrant circuits.
For low voltage electrograms in a broad area, particularly
in the left atrium, no surgery is performed, because
of the low rate of converting AF.
|
|
|
Of 45 patients, 3 had low voltage electrograms in
the broad left atrium area, and AF surgery was not
indicated (Figure
5). In the remaining 42 patients, 8 patients clearly
demonstrated passive activation in the right atrium
combined with focal activations in the left atrium.
The PV isolation was performed in these patients,
and sinus rhythm resumed in all. In the remaining
34 patients, reentrant activation was observed in
the right atrium and focal activation in the left
atrium. The radial procedure was performed, with a
91% success rate.
|
|
Conclusions
Intra-operative mapping is useful to determine the
optimal procedure in each patient.
In patients with passive activation in the right atrium
without reentrant or focal activations, simplified
procedures confined to the left atrium or the PVs
may be indicated. However, ablation of a single focus
may fail to cure AF because there can be more than
one source of focal activation and the second focus
would assume dominance after the ablation of the primary
focus.
The limitations for the present system for intra-operative
mapping include the lack of data from the septum,
the effect of anesthesia, the possibility that the
activation pattern may vary, and the considerable
time to analyze the data and determine the activation
pattern. A solution may be pre-operative catheter
mapping. Right atrial endocardial mapping using a
basket catheter or non-contact balloon catheter electrode
may determine whether the right atrial activation
pattern is passive or reentrant. Within five years,
it is likely that mapping or a electrophysiologic-guided
procedure will be established. The procedure will
be minimally invasive, probably using a robot without
the use of cardiopulmonary bypass.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2004
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|