 |
|
|
 |
| Regeneration Medicine in Cardiovascular Diseases |
|
|
|
 |
|
Embryonic
Stem Cell Differentiation to Cardiomyocytes
Richard Lee
Brigham and Women’s Hospital,
Boston, MA
|
|
Embryonic stem cells and tissues are derived from
the inner cell mass of the blastocyst. The embryonic
stem field is unique because under highly-specialized
conditions the cells will continue to proliferate
and be multi-potent. Although these highly-specialized
conditions are very useful in the laboratory, they
are also confining; the specialized manner in which
the cells are grown restricts the ability to steer
them down one pathway versus another pathway.
The typical manner in which embryonic stem (ES)
cells have been differentiated involves their being
cultured on embryonic fibroblasts or treated with
leukemia inhibitory factor, which maintains the cells
in the undifferentiated state. A key distinction for
human stem cells is that they are not maintained in
the undifferentiated state by leukemia inhibitory
factor. The secreted product from human ES cells that
allows them to be undifferentiated is not known.
In the classic differentiation protocol, a hanging
drop is the most typical way for aggregating these
cells. The cells are suspended and aggregated in a
small cluster for a number of days and form the embyroid
bodies, which can be plated on a monolayer-type dish,
after which the cells will differentiate.
Another question is what is the embryoid body. Although
it is thought that these cells talk with each other
to promote differentiation, there is little understanding
of why this process occurs. The typical efficiency
of plating the embryoid body is quite low for obtaining
aggregates of ES cells, a little less than 1%. ES
cells have a tendency to differentiate to a neuronal
phenotype, for reasons that are not really understood.
To drive the efficiency of the differentiation process,
Lee and colleagues used the myosin heavy chain promoter
and made a stem cell line that reported with green
fluorescent protein. The concordance was very high,
showing this to be an easy way to pick out cells that
had differentiated to a cardiac myocyte from large
numbers of samples. Using a number of different markers,
including sarcomeric myosin and sarcomeric alpha-actinin,
it was confirmed that these were cardiac myocytes.
In a trial run in which 800 compounds were screened
in a drug library, Vitamin C was the only positive
compound that reproduced. It is unknown why the ES
cells in monolayers exposed to increasing concentrations
of ascorbic acid resulted in about a 5-fold increase
in the amount of cardiac myocytes in an ES culture.
This is still not highly efficient, it can reach about
5-6% differentiation. It does not seem to be related
to the anti-oxidant properties of ascorbic acid. Vitamin
C has a complex mode of action that still is not elucidated
after 30 years of research.
The ascorbic-induced GFP+ cells were positive for
sarcomeric alpha-actin. In the presence of ascorbic
acid, it drove the myocyte formation with a number
of markers, including sarcomeric actin and myosin.
Ascorbic acid also drove the formation of the gene
expression for ANF, beta myosin heavy chain, alpha
myosin heavy chain, Nkx2.5, and GATA4, all of which
were confirmed by real-time PCR.
This was a curious finding, because it meant that
a single compound can shift embryonic cell differentiation.
It is challenging to determine why this might happen,
when considering the cascade of events that are required
for cardiogenesis in the developing embryo. It is
becoming quite clear that relatively simple manipulations
of ES cells can steer cells from one pathway down
another pathway, including the cardiogenesis pathway.
Some factors that can drive cardiogenesis include
embryoid body formation, TGF-beta family members (IGF-1,
FGFs, Oxytocin, EPO, retinoic acid, DMSO, dynorphin),
co-culture with visceral-endodermal cells, electrical
stimulation, hydrogen peroxide, and some new compounds.
Some people are purporting the ability to convert
90% of ES cells into cardiac myocytes, but this data
is very new and needs to be confirmed in many laboratories
to ensure it is a general effect on many different
types of ES cell lines.
In spite of the complexity of developmental programs
that causes a cardiomyocyte to form in vivo, which
includes signals of BNP pathways, FGF pathways, among
others, and the necessity for signals to turn on and
off, it is interesting that some relatively simple
manipulations in culture can allow for differentiating
stem cells.
Many large questions remain in cardiac regeneration.
A key question is whether or not it is possible to
recruit endogenous cells or whether it will be necessary
to inject cells. For cell therapy, will human ES cells
be needed? Strategies for rejection must be studied.
Will there be a need for universal donor cells, or
will some stem cells be permissive with regard to
rejection? These questions must be answered before
stem cells can be used therapeutically.
Perhaps the greatest challenge ultimately will be
that injecting cells and getting them to live may
not lead to long-term function of the heart. In all
species there is a very fine vascular structure that
is very intimately related to the myocytes. Ultimately,
because of the problems of oxygen diffusion in the
heart, and probably other factors, it is necessary
to achieve a 3-dimensional structure of the myocardium
that will need to be restored by regenerative strategies.
An encouraging factor is that cardiac myocytes seem
to have some intrinsic information that tells them
to form this relation between the myocyte and the
vasculature. When mixing cardiac myocytes with endothelial
cells, they will spontaneously form vascular structures,
as endothelial cells do in 3-dimenstional cultures.
Then the myocytes will wait for the vascular structures
to form and then grow on the outside. It looks very
much like a process similar to vasculogenesis. Lee
and colleagues believe thatmyocytes have some instructions
that allow them to help form this structure. However,
with regenerative strategies it will be important
to ensure that the myocytes retain those same instructions.
An injected cell will need to find its correct location
relative to the vasculature or it will have to create
its own vasculature. Ultimately, getting cells to
survive is the beginning, and more research will be
needed on the end structural solution.
|
PAGE
TOP
|
Regeneration
Therapy for Heart Failure Due to Nonischemic Cardiomyopathy
Genzou Takemura
Gifu University School of Medicine,
Gifu, Japan
|
|
Beneficial effects of treatment with autologous bone
marrow cell (BMC) transplantation in doxirubicin cardiomyopathy
in rabbits and of treatment with granulocyte-colony-stimulating
factor (G-CSF) in hereditary cardiomyopathy in hamsters
(Um-X7.1) was shown in work by Takemura and colleagues.
The beneficial effects on nonischemic chronic heart
failure (CHF) were accompanied by prevention of myocyte
degeneration and death, activation of MMPs, suppression
of TNF-aalpha, and tissue regeneration.
The results provide evidence that bone marrow implantation
and G-CSF may be a novel therapeutic strategy for
heart failure due to nonischemic cardiomyopathy.
|
|
Doxirubicin cardiomyopathy in rabbits
|
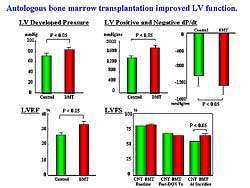 |
| Figure
1. Left ventricular function was improved by autologous
bone marrow transplantation in this animal model.
|
| Click
to enlarge |
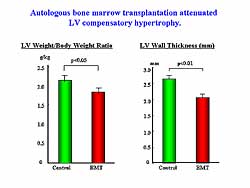 |
| Figure
2. Left ventricular hypertrophy was attenuated
by autologous bone marrow transplantation. |
| Click
to enlarge |
|
The cardiomyopathic model was created by an 8-week
intravenous injection of doxirubicin into rabbits.
Bone marrow cells (BMCs) were aspirated from the iliac
bone 2 weeks later and mononuclear cells were directly
injected into about 10 points of the left ventricular
(LV) wall. Hemodynamic and histological assessments
and molecular biological assay were performed 4 weeks
later.
Parameters of LV function, LV developed pressure,
positive and negative dP/dT, ejection fraction, and
fractional shortening, were significantly improved
by the BMC implantation (Figure
1). LV compensatory hypertrophy was significantly
attenuated by the BMC implantation, as shown by a
smaller heart to body weight ratio and a thinner LV
wall thickness (Figure
2).
The percentage of fibrotic area was significantly
reduced in the BMC implantation group. Extracellular
matrix metalloproteinases (MMP-2, MMP-9) were significantly
increased in the hearts treated with BMC. The cardiotoxic
cytokine, TNF-alpha, was significantly reduced on
Western blot in the BMC implantation group.
|
|
Hereditary cardiomyopathy in hamsters
G-CSF can proliferate and differentiate granulocytes,
and also release bone marrow stem cells into peripheral
blood flow. This group recently reported that treatment
with G-CSF after acute myocardial infarction improved
cardiac function. However, it is unknown whether G-CSF
has a beneficial effect in nonischemic heart failure,
such as dilated cardiomyopathy (DCM), a major cause
of morbidity and mortality in CHF. Thus, this group
examined the long-term effects of G-CSF on survival,
cardiac function, and cardiac histology in the hamster
model of nonischemic heart failure.
The UM-X7.1 hamster is a representative animal model
of autosomal recessive cardiomyopathy and vascular
dystrophy. Because of the lack of the delta-sarcoglycan
gene, it develops progressive myocyte death beginning
at about 4 weeks of age and worsens over time. At
20 weeks, cardiac hypertrophy is seen, followed by
progressive ventricular remodeling, myocardial fibrosis,
and CHF. About 50% of these animals die by 30 weeks
of age. Importantly, 1 family and 2 sporadic cases
of human DCM were identified who presented with mutations
in the delta-sarcoglycan gene, comparative to this
animal model.
Intraperitoneal injection of G-CSF (10 mg/kg/day)
was started at 15 weeks of age in male UM-X7.1 hamsters
(n=16) and continued until 30 weeks of age. In the
control group (n=15) the animals were treated with
the same amount of distilled water in a similar manner.
In other experiments with mice, this group already
confirmed that cardiac myocytes under pathological
conditions express G-CSF receptor.
|
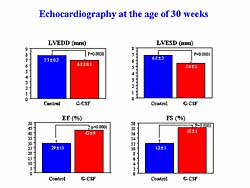 |
| Figure
3. Treatment with G-CSF improved left ventricular
function at 30 weeks in the animal model. |
| Click
to enlarge |
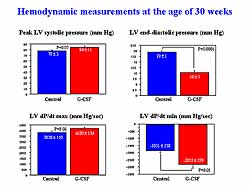 |
| Figure
4. Treatment with G-CSF improved hemodynamic measurements
at age 30 weeks. |
| Click
to enlarge |
|
Survival at 30 weeks was significantly improved with
G-CSF treatment compared to controls (100% versus
53%, respectively; p<0.0001). The surviving animals
underwent echocardiography at 30 weeks of age. The
control hamsters showed severe LV enlargement and
signs of decreased cardiac function, a low LVEF and
percent fractional shortening, while each of these
was significantly improved in the G-CSF treated animals
(Figure
3).
Similarly, cardiac catheterization revealed significantly
improved LV function and congestion in the G-CSF treated
animals versus controls (Figure
4). The heart was smaller in the G-CSF treated
animals at necropsy. The heart to body weight ratio
was significantly smaller in the G-CSF treated animals.
Histologically, there were numerous foci in the fibrotic
area and abundant interstitial fibrosis in the untreated
hearts. Fibrosis in the ventricles was significantly
reduced in the G-CSF treated hearts. The percent area
of fibrosis in the ventricles was 8.6% in the treated
animals versus 20% in the untreated animals at 30
weeks. The percent area of fibrosis was 7.3% in animals
at the age of 15 weeks before treatment, which was
not significantly different from that of the G-CSF
treated animals at 30 weeks of age.
|
|
Electron microscopy analysis of the heart revealed
degenerative changes of the myocytes with various
grays in the untreated group. Many myocytes in the
untreated hearts showed severe vacuole degeneration.
The vacuoles contained degraded subcellular organelles,
such as mitochondria. However, these were greatly
attenuated in the G-CSF treated animals. In another
example, the cardiac myocyte was affected by severe
autophagic degeneration with scanty myofibrils in
the periphery. It is assumed that such a cell can
no longer function normally, but it is unknown whether
such degeneration is linked to cell death.
Thus, sarcolemma integrity was analyzed by peritoneal
injection of Evans blue dye 24 hours before sacrificing
the animals. The dye was exuded by cardiomyocytes
that preserve normal sarcolemma permeability, but
is taken up by the cardiomyopathic cells with leaky
cell membrane. Such cells can be recognized to be
dead. The control hearts revealed extensive dye uptake
by cardiomyocytes, while the uptake was significantly
ameliorated in the G-CSF treated hearts.
Immunohistochemistry revealed a significantly lower
incidence of cardiac myocytes positive for cathepsin
D and for ubiquitin in the G-CSF treated hearts than
in the control hearts. Cathepsin D and ubiquitin are
markers of autophagy. Under confocal microscope, cathepsin
D positive cells were found that were taking Evans
blue dye simultaneously. This suggests continuity
between autophagic degeneration and autophagic cell
death. Overall, these findings indicate a protective
effect of G-CSF against autophagic degeneration and
death of the cardiomyocytes.
The possibility of myocardial regeneration was checked
in the present model. Bone marrow was aspirated from
the femur bones of 15-week-old hamsters and labeled
with fluorescent dye and autologously returned into
the bone marrow spaces. The animals were treated with
G-CSF or distilled water for 2 weeks. Cells that were
double positive for cells that were muscle-specific
troponin I and DiI, which are considered BMC-derived
cardiomyocytes, were found, although the incidence
was small (<0.1%) in the G-CSF treated hearts,
but none in the untreated hearts. However, they could
not find DiI-labeled endothelial cells or vascular
smooth muscle cells in the hearts of either group.
The present study revealed a significant reduction
of fibrosis in the G-CSF treated hearts. They noted
a significant increase MMP-2 and MMP-9 in the G-CSF
treated hearts, compared with the control hearts.
TNF-alpha is not only one of the representative toxic
cytokines that can directly depress cardiac function,
but also contributes to angiotensin II-mediated fibrosis
of organs through upregulation of angiotensin II type-1
receptor. In the present model, they found that long-term
therapy with G-CSF resulted in a significant reduction
in the cardiac TNF-alpha level in the 30-week-old
hamsters. Overall, such an increase in MMP activity
and TNF-alpha downregulation in the heart caused by
G-CSF might have contributed to thereduction of collagen
content in the G-CSF treated hearts.
|
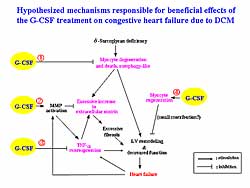 |
| Figure
5. The hypothesized mechanisms responsible for
the beneficial effects of G-CSF treatment heart
failure due to DCM |
| Click
to enlarge |
|
The hypothesized mechanisms for responsible for the
beneficial effects of the G-CSF treatment on CHF due
to DCM are shown in Figure
5. The first mechanism is protection of cardiomyocytes
from autophagic degeneration and death. The second
mechanism, MMP upregulation, would cause degradation
of excessive extracellular matrix and reduction in
ventricular fibrosis, and then may reduce wall stiffness
and contribute to improvement of cardiac function.
The third mechanism, TNF-alpha downregulation, would
relieve cardiotoxic action of TNF-alpha on the heart
and may contribute to functional improvement. The
fourth mechanism is cardiomyocyte regeneration would
result in preservation of the contractile force of
the myocardium.
|
PAGE
TOP
|
Application
of Embryonic Stem Cells for Vascular Regeneration
Medicine
Hiroshi Itoh
Kyoto University School of Medicine,
Kyoto, Japan
|
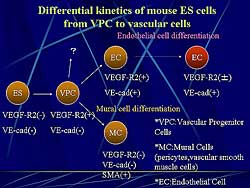 |
| Figure
1. The differential kinetics of mouse embryonic
cells from vascular progenitor cells to vascular
cells. |
| Click
to enlarge |
|
Previously this group demonstrated that VEGF-R2 (Flk1)-positive
cells derived from mouse embryonic stem cells (ES
cells) can differentiate into both endothelial cells
and mural cells and organize into blood vessels in
vitro and in vivo. They called these cells vascular
progenitor cells (VPC). They have also demonstrated
that implanted VPC derived from mouse ES cells could
be incorporated in tumor vessels and augment tumor
blood flow in a tumor angiogenesis model, suggesting
the therapeutic potential of VPC for vascular regeneration.
Figure
1 shows the differential kinetics of mouse ES
cells from VPC to vascular cells. VEGF-R2(-)and VE-cad
(-) undifferentiated ES cells differentiate into VEGF-R2(+)
VE-cad(-) VPC. VPC can differentiate into VEGF-R2(+),
VE-cad (+) endothelial cells. In contrast, the same
VPC can differentiate into VEGF-R2(-) VE-cad(-) and
alpha smooth muscle actin positive (SMA) mural cells.
|
|
Study design
To investigate the potential of ES cell-derived
VPC for clinical application, they examined whether
VPC occur in the primate cynomolgus monkey and human
ES cells.
ES cell markers in mice ES cells are SSEA-1(+) SSEA-3(-)
SSEA-4(-) TRA1(-), while in primates they are SSEA-1(-)
SSEA-3(+) SSEA-4(+) TRA1(+). In terms of morphology,
mice ES cells grow in rounded clumps with indistinct
cell borders and primate ES cells grow in flat colonies
with distinct cell borders. Regarding l eukemia inhibitory
factor (LIF) deficiency, in mice ES cells pluripotent
cells can remain undifferentiated, but not in primate
ES cells. Even on a feeder cell layer, all primate
pluripotent cells grow very poorly when dissociated
to single cells, whereas mouse ES cell lines can be
cloned with relatively high efficiency in the presence
of LIF. There are also differences in attachment to
extracellular matrix.
|
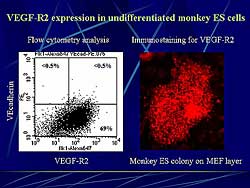 |
| Figure
2. VEGF-R2 expression in undifferentiated monkey
ES cells. |
| Click
to enlarge |
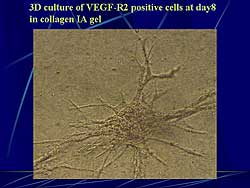 |
| Figure
3. 3-dimensional culture of VEGF-R2 positive cells
at day 8 in collagen IA gel. |
| Click
to enlarge |
|
In contrast to mice ES cells, monkey undifferentiated
ES cells express VEGF-R2. Flow cytometry analysis
revealed that about 70% of ES cells were positive
for VEFF-R2 (Figure
2). On immunostaining, ES cells colonies cultured
on MEF layer were stained positively for VEGF-R2.
On flow cytometry, VEGF-R2 expression was detected
in about 70% of undifferentiated monkey ES cells at
day 0, but VEGF-R2 expression diminished by day 6
of differentiation on OP9 feeder layer. VEGF-R2 expression
re-appeared in about 8% of the total cells after 8
days of differentiation. At day 10, VE-cad(+) endothelial
cells appeared.
To compare monkey VEGF-R2(+) cells at day 0 and
day 8, alkaline phosphatase activity, which was reported
to be detected in undifferentiated ES cells, was examined.
Alkaline phosphatase activity was clearly detected
in undifferentiated monkey ES cells at day 0, but
not cells at day 8 cultured on OP9 feeder layer.
The re-culture of VEGF-R (+) monkey ES cells at
day 0 did not differentiate into PECAM1 positive endothelial
cells after 5 days of re-culture on OP9 feeder layer.
At day 8, VEG-R2(+) cells differentiated into PECAM1,
VEcadherin, and eNOS positive cells after 5 days of
re-culture on OP9 feeder layer. When re-culturing
on a collagen IV coated dish with 10% serum for 5
days, the VEGF-R2(+) cells differentiated at day 8
into alpha-SMA, calponin, and smooth muscle myosin
heavy chain positive cells. The addition of VEGF-R2(+)
cells to culture on collagen IV coated dish resulted
in the appearance of PECAM1 positive cells that were
surrounded by SMA positive cells. A 3-dimensional
culture of VEGF-R2(+) cells at day 8 in collagen IA
gel showed aggregates from which cells migrated outward
and formed tube-like structures within 3 days.
Human ES cells were positively stained for SSEA4
and were positive for alkaline phosphatase activity.
VEGF-R2(+) cells in human ES cells at day 0 were positive
for TRA1-60 activity, but during differentiation on
OP9 feeder layer VEGF-R2(+) TRA1-60(-) cells appeared.
On re-culture, the endothelial cell marker CD34, VEcadherin,
PECAM1, and eNOS positive cells appeared after 8 days
re-culture of TRA1-60(-) VEGF-R2(+) VEcadherin(-)
cells on a collagen IV coated dish with 10% serum
and VEGF 50 ng/ml. PECAM1 negative cells were positively
stained for alpha-SMA. When cultured without VEGF,
nearly all of the cells differentiated into alpha-SMA
positive and calponin positive mural cells. A 3-dimensional
culture of VEGF-R2(+) TRA1-60(-) cells in collagen
A1 gel at day 8 showed that undifferentiated VEGF-R2(+)
cells could not aggregate on the floating culture,
unlike the mice and monkey cells (Figure
3). They mixed gene cells in collagen A1 again
and re-cultured, after which some of the cells formed
tube-like structures within 5 days.
|
|
Conclusion
Their findings indicate that VEGF-R2(+) cells differentiated
on the OP9 feeder layer can act as vascular progenitor
cells in primates. Further, the differentiation kinetics
of VPC in the primate and mice ES cells are different.
They identified VPC in primates. The VPC-derived cells
can be highly expanded in vitro, and would be a promising
material for vascular regeneration therapy.
|
PAGE
TOP
|
Three-Year
Follow-Up of the Safety and Feasibility of Intramyocardial
Bone Marrow Mononuclear Cell Implantation in Patients
with Ischemic Heart Disease
Kimikazu Hamano
Yamaguchi University School of
Medicine, Ube, Japan
|
|
Bone Marrow Cell Implantation (BMCI) has been performed
by Hamano and colleagues since 1999. Focused cell
sources include bone marrow mononuclear cells (BM-MNCs),
peripheral blood (EPC, CD34 cells), umbilical blood
(EPC, CD34 cells), and ES-derived cells (EPC, EC,
SMC). BM-MNCs were used in their work because they
consist of many endothelial progenitors and cytokine-producing
cells, without the problems of immunological rejection
and ethical conflict for clinical application.
The mechanisms of therapeutic angiogenesis by BMCI
include the induction of angiogenesis by cytokine-producing
cells. Endothelial progenitor cells induce vasculogenesis,
resulting in new collateral vessels result. However,
the process of vasculogenesis is not clarified, and
the contribution of angiogenesis and vasculogenesis
are not clearly defined.
|
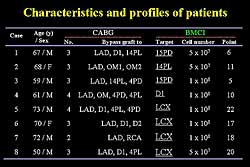 |
| Figure
1. Characteristics and profiles of the 8 study
patients. |
| Click
to enlarge |
|
The inclusion criteria were patients with severe
ischemic heart disease (IHD) scheduled to undergo
coronary artery bypass grafting (CABG), at least 1
perfusion that showed ischemia and coronary artery
stenosis that was unsuitable for traditional PCI or
CABG, and no wall thinning of an old myocardial infarction.
BM-MNCs were prepared and then injected into ungraftable
vessels. To date, BMCI has been used in 8 patients,
ranging in age from 50 to 73 years, who had 2-4 grafts
performed. Figure
1 shows the patient characteristics. Initially,
the target area was the small area of the left circumflex,
but now the total circumflex area is treated. The
number of cells initially was 5x107 and
is now 5x108.
|
|
The targeted perfusion area was improved in 5 of
the 8 patients. Wall motion of the target area was
improved in 2 of 8 patients. In the initial 4 cases,
only a small area of the circumflex was treated, therefore
it is difficult to judge improvement in left ventricular
(LV) function.
In a representative case, the coronary angiogram
showed no graftable vessels in the circumflex area,
and tight stenosis in the left anterior descending
(LAD) (Figure
2). The pre-operative echocardiography showed
reduced wall motion in the circumflex. The LAD was
bypassed and cells injected into the circumflex area.
After treatment, wall motion in the circumflex area
was improved. Post-operative scintigraphy showed no
ischemia in the circumflex area. The coronary angiogram
after treatment showed right filling of the circumflex
area. Scintigraphy performed 2 years after treatment
showed no ischemic change in the circumflex area (Figure
3).
In another case, before surgery several stenoses
could be seen in the LAD and no graftable vessels
in the circumflex area. Scintigraphy showed ischemia
in the anterior and lateral walls, indicating the
posterior wall was already dead. After treatment,
no ischemia was seen in the anterior or lateral walls.
The LAD was bypassed and cells injected into the circumflex.
Scintigraphy at 1 year after treatment showed no ischemia
in any part of the left ventricle. Pre-operative left
ventriculography showed reduced wall motion in the
circumflex area, however, after treatment the wall
motion was improved.
To quantify the perfusion of the circumflex, they
measured the perfusion index at the circumflex area
and compared this to the control group, comprising
patients who did not have bypass performed in the
circumflex area. In the BMCI group, the perfusion
index in the circumflex increased after treatment,
while it was not changed or reduced in the control
group.
To evaluate the safety of this treatment, mass formation
or calcification was assessed by CT, chest radiography,
and echocardiography. Through the first 3 years, no
abnormality was found in any patient. Also, no significant
or lethal arrhythmias were found in any patients.
There was PVC in 1 patient. At 3 years follow-up,
no significant arrhythmias had occurred.
In summary, remarkable improvement of regional perfusion
and clinical symptoms were observed in about 70% of
patients after BMCI treatment, which continued throughout
the 3-year follow-up period. No systemic or local
side effects related to BMCI treatment was recorded
by the 3-year follow-up examinations.
|
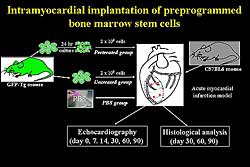 |
| Figure
4. Intramyocardial implantation of preprogrammed
bone marrow stem cells. |
| Click
to enlarge |
|
Can BMCI regenerate injured myocardium effectively?
They cultured BMC with a low concentration of TGF-beta,
so the cell shape was changed somewhat and it expressed
myosin. BMC were harvested from GFP-transgenic mice,
which were pre-treated with TGF-beta (Figure
4). Acute myocardial infarction models were then
injected with pre-treated BMC, untreated BMC, or PBS
alone. LV function, echocardiography, and histology
were assessed. In the pre-treated group, cells survived
and expressed myosin. However, in the untreated group,
some cells survived but did not express myosin. In
the pre-treated group, the percent fractional shortening
was significantly improved compared to the untreated
group (Figure
5). Therefore, they conclude that BMCI should
be a feasible and safe method to induce therapeutic
angiogenesis for the treatment of ischemic heart disease,
and is also a possible method for regenerating injured
myocardium.
|
PAGE
TOP
|
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2004
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|