|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Myocardial Angiogenesis: Protein and Gene TherapyTimothy D. HenryMinneapolis Heart Institute
|
Refractory angina is associated with morbidity and decreased quality of life, and its treatment options include optimal medical management, optimal risk factor modification, revascularization, external counterpulsation (ECP), and myocardial angiogenesis.
The objective of growing new blood vessels is to increase collateral vessel growth, either by angiogenesis or by arteriogenesis (vessels < 200 microns). However, even at their best, the new blood vessels are unlikely to be as good as normal epicardial vessels, and for example, it will not be possible to insert a drug-eluting stent into such a vessel.
Angiogenesis works very well in preclinical models, using protein therapy, gene therapy, and cell therapy, in many different models, including chicken embryos, rabbit corneas, rabbit hindlimbs, porcine ameroid models. The successful Phase I trials have caused much excitement. A variety of endpoints, related to symptoms and myocardial function, were improved in Phase I unblinded trials of protein therapy, gene therapy, and cell therapy.
Results of angiogenesis in placebo-controlled protein trials
|
Although the field of angiogenesis is nearly a decade old, relatively few trials have been conducted: 4 protein trials (2 coronary bypass) using intracoronary VEGF protein and intracoronary FGF-protein, and 4 gene therapy trials, using intracoronary adenovirus encoding for fibroblast growth factor-4 (Figure 1). Despite the excitement about cell therapy, no placebo controlled trials have been reported, although one was completed recently.
The VIVA trial
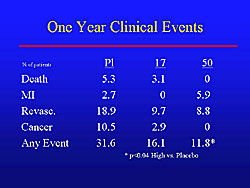
The VIVA trial was the first randomized, double-blind, placebo-controlled trial of an angiogenic growth factor. The primary endpoint of exercise time at 3 months was negative. Notably, however, exercise time continued to decrease in the placebo group, while the treatment group continued to improve over time. A significant improvement in angina was seen at the end of the trial, and a strong trend for an increase in exercise time in the treatment group (45 seconds versus 24 seconds). The clinical results at 1-year are shown in Figure 2. The combined endpoint at 1 year was 31.6% in the placebo group versus 11.8% in the high-dose treatment group (p<0.04). More cancer occurred in the placebo than in the treatment groups, illustrating another important reason for placebo groups. Mortality was low at 1 year, although not statistically significant, they trended in the right direction with no deaths in the high-dose group, 3.1% in the low-dose group and 5.3% in the placebo group.
In the VIVA trial, treatment was safe and well-tolerated, with a very low 1-year mortality rate and MI rate. The placebo effect had completely worn out by 1 year. There was persistent benefit in the treated patients, especially in the high-dose group. There was less revascularization and less occurrence of the composite endpoint. So, even though the primary endpoint was negative, the secondary endpoints were encouraging and showed there was likely some physiologic effect.
The FIRST Trial
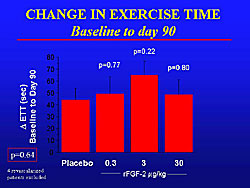
The FGF-2 Initiating Revascularization Support Trial (FIRST) was the first randomized, double-blind, placebo-controlled study of intracoronary FGF protein. A total of 337 patients were randomized to placebo or 3 different doses of intracoronary FGF-2 (0.3 mg/kg, 3.0 mg/kg, or 30mg/kg). The primary endpoint of change in exercise tolerance was negative (Figure 3). The placebo patients had about a 45 second improvement in exercise time. However, a significant improvement in angina in each treatment group, but was most pronounced in the 3 mg/kg group.
|
|
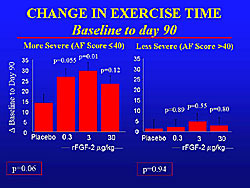
In patients with high angina frequency scores on the Seattle Angina Questionnaire (SAQ), a trend for a reduction in angina frequency was seen in each group, which was statistically significant in the 3 mg/kg group (Figure 4). In contrast, those with lower SAQ scores at baseline did not have a significant difference.
Much data show that progenitor cells, growth factors, and VEGF receptors decrease with age. But, several trials have shown that older patients may actually show more benefit. This is likely because they have more ischemia, making it easier to show a change. In the FIRST trial, a significant improvement in exercise time was seen in the treated versus the placebo patients over 63 years of age, whereas in the patients under 63 years, there was no significant improvement.
Results of angiogenesis in placebo-controlled gene therapy trials
The AGENT trials
The AGENT 1 and 2 trials were small trial that demonstrated the feasibility and safety, and laid the groundwork for the largest, double-blind, placebo-controlled gene therapy, the AGENT 3 trial.
In AGENT 3, 79 patients with stable angina were randomized to placebo (n=19) or to increasing intracoronary doses of adenovirus encoding FGF-4 (Ad5FGF-4). No improvement was seen for the primary endpoint of change in exercise time from baseline at 4 and 12 weeks and 6 months.
|
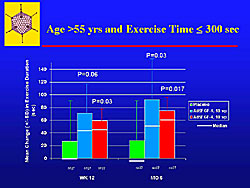
In patients with at least a 30% improvement, to look at responders, there was no significant improvement in exercise time. In older patients, over 55 years, a significant improvement in exercise time was seen; the placebo effect was less prominent in the older patients (Figure 5).
In the high-risk patients with class III or IV angina and over 55 years old, at week 12 and 6 months, there was more improvement compared to the low risk patients, which was statistically significant in the high-dose group and a trend in the low-dose group. Hence, by focusing on the high-risk groups in these trials, it may be possible to show improvement in exercise time.
A low 1-year event rate for the composite endpoint was found (death, nonfatal MI, and myocardial ischemia leading to hospitalization or revascularization. Mortality at 1-year was low, at only 1%. This again highlights that these patients suffer mostly from morbidity. The rate of nonfatal MI was also low at 1-3%. A significant number of people (14-17%) were admitted to hospital for either revascularization or angina, although this was not statistically significant.
The final results of the AGENT 2 and 3 trials are expected to be available in mid-2005.
|
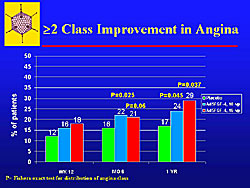
A Fishers distribution of angina showed that a significant number of patients had a greater than 2 class improvement, and there were fewer patients who had worsening of angina (Figure 6).
At 1-year, gene therapy reduced chest pain. There is a consistent pattern of improvement in chest pain and yet no change or only trends for improvement in exercise time. For exercise time, a decrease was seen in the placebo patients, and a 70-80 second improvement in the two treatment groups. All were high-risk patients (class III or IV angina) patients.
Improvement in exercise time can be found with gene therapy, when there is careful patient selection and careful control of the stress test.
Intramyocardial delivery of gene therapy
Although intracoronary delivery is easier, many believe that intramyocardial delivery has significant advantages.
Interesting data have come from two pilot trials of intramyocardial gene therapy delivery. In a trial of 9 patients, an initial improvement was seen all patients, but when the placebo effect wore off, the treatment effect continued to improve, and at 1 year there was a significant, but small, improvement.
The second placebo-controlled trial with 19 patients was stopped early, but will continue in a trial called the GENESIS trial, with increasing doses. No clear dose response has been seen so far in the GENESIS trial. In the pilot trial of high-risk patients, however, a significant improvement in exercise time in the treatment group and none in the placebo patients.
In the EUROINJECT trial in patients with severe ischemic disease, intramyocardial delivery of phVEGF-A165 was performed. The primary endpoint of change in SPECT imaging was negative, with 30% of the placebo and 40% of the treated patients having an improvement in SPECT imaging (NS). However, within the treated group, there was a statistically significant improvement.
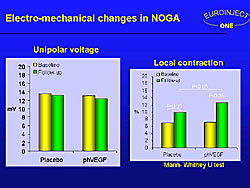
A significant improvement in local contraction was seen in both groups, using NOGA electro-mechanical mapping, with a statistically significant improvement in the treated group compared to the placebo group (Figure 7).
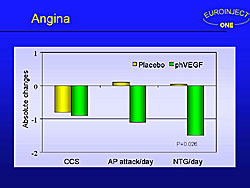
EUROINJECT trial is the 5th placebo-controlled trial to show a reduction in angina with treatment, despite the negative primary endpoint. In this trial, there was an improvement in the number of angina attacks per day and in the number of nitroglycerin tables used per day (Figure 8).
|
|
Why is there ongoing improvement?
In the AGENT-3, looking at endothelial progenitor cells (EPC), particularly CD34+ cells, no change was seen in the placebo group, whereas at 3 and 4 weeks a significant increase in the circulating EPC in the treated group. This provides some evidence to support the hypothesis that gene therapy itself may stimulate an increase in EPC, which may be part of the reason for the late or ongoing improvement in these placebo-controlled trials.
The REVASC trial
In this Phase II, multicenter trial, patients had Class II-IC angina and were randomized to intramyocardial injection of AdVEGF121 via a minithoracotomy (30 direct injections in the free wall of the left ventricle) versus ongoing medical therapy. This was a 26-week study with 52 weeks of safety follow-up. Although this is not a placebo-controlled trial, it is important for providing an histology. An improvement in exercise time and a reduction in angina were found in this trial, which is consistent with the hypothesis of improving neovascularization. Notably, evidence of neovascularization was seen in the left ventricle of a patient who died of an MI, unrelated to the gene therapy, in the post-operative period.
Summary of placebo-controlled angiogenesis trials
To date, a total of 1229 patients have been studied in placebo-controlled trials of angiogenesis, using 7 different agents and methods of delivery. Myocardial angiogenesis appears to be safe, and pre-specified blinded secondary endpoints have been positive. A reduction in angina has been shown in these trials. Post-hoc analyses show that high-risk patients may have more benefit. However, the efficacy has been modest. The patients are challenging, in whom conventional therapies have not bee successful (bypass surgery in 80% of the studied patients, angioplasty in about 75%, and optimal medical therapy).
None of the therapies to date have improved exercise time, including myocardial angiogenesis, EECP, TMR/PMR, neurostimulation (recommended by the European Society of Cardiology), or novel drugs.
Lessons learned include a prominent placebo effect about which more needs to be learned; there is excellent short term safety; the concerns about cancer and atherosclerosis have not been seen; 3 to 6 months seems to be the best time point for measuring the effect, both for the placebo effect to wear off and the treatment effect to increase; the endpoint is a major issue, an ideal endpoint for assessing myocardial blood flow has not been found and this is a major limitation in the field. It is very hard to make healthy patients better, and the focus should be on the highest risk patients.
What remains to be learned? The ideal growth factor or combination (HIF-1, HGF, EPC, bone marrow, stem cells) needs to be tested in a careful, controlled manner, as well as the ideal dose and route of administration. Also, determining which patients are responders and non-responders, the optimal time point for assessment of endpoints, and what are the best investigative surrogate endpoints. In 5 to 10 years, Henry sees there will be a targeted treatment strategy that identifies the patient’s defect in collateral development and uses a treatment for that particular defect.
Ongoing angiogenesis trials
The ADVANCE phase I trial with 24 patients will give GCSF (5 mcg/kg/day x 5 days) and then apheresis is performed to select the CD34+ cells, and then inject the cells intramyocardially using NOGA mapping into the areas of myocardial ischemia. This is a double-blind trial with 3:1 randomization and crossover of placebo patients using frozen cells at 6 months.
In the GENASIS double-blind, placebo-controlled trial, 404 patients will be randomized to placebo, or three different doses ((20 ug, 200 ug, 800 ug) of an intramyocardial injection of plasmid for VEGF-2 using a Stilleto catheter. The primary endpoint is exercise tolerance at 3 months.
Therapeutic Angiogenesis Using HGF (Hepatocyte Growth Factor) Gene to Treat Ischemic DiseaseRyuichi MorishitaOsaka University
|
Intramuscular injection of angiogenic plasmid has been shown to stimulate collateral formation in patients with peripheral arterial disease (PAD). Morishita and colleagues used hepatocyte growth factor (HGF) in pre-clinical studies as a potent angiogenic growth factor, because it was originally cloned as the most important mitogen to stimulate hepatocytes. HGF belongs to the Kringle family, which also includes t-PA and urokinase.
In their work, collateral vessel formation was stimulated by HGF gene therapy in various animal models, including diabetes, atherosclerosis, and hyperlipidemia). All of their preclinical studies demonstrated that HGF has potent angiogenic factors, even in high-risk conditions.
TREAT-HGF trial
Based on their pre-clinical findings, Morishita and colleagues conducted the open-label TREAT-HGF trial to stimulate collateral formation in 22 patients with peripheral arterial disease (PAD). Intramuscular injection of naked plasmid DNA was performed. The primary endpoint was safety of HGF gene therapy, and secondary endpoints were the bioactivity of gene therapy and defining the effective dose.
|
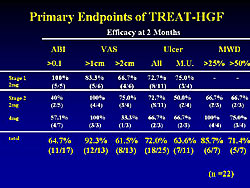
At 2 months, improvement > 0.1 in the ankle brachial index (as a functional endpoint) was found in 11 of 17 (64.7%) patients. The visual analog scale (VAS), assessing resting pain, was improved by > 2 cm in 61.7% of patients. An improvement > 25% in ischemic ulcer was found in 18/25 (72%) of patients (Figure 1). For the combination of functional and clinical endpoints, 18 of 22 patients showed an improvement in at least 1 endpoint at 2 months.
At 2 years, the majority of the adverse events were mild and related to pain at the injection site. There were 27 mild adverse events that were definitely related and 189 mild events that were possibly related to gene therapy. Four severe events and 1 moderate event was possibly related to gene therapy. There were no serious adverse effects related to gene therapy, nor edema, hemangioma, tumor, or diabetic retinopathy.
They concluded that HGF therapy might be safe and well tolerated over the 2-year follow-up.
Serum HGF protein levels were not increased, even after the second injection. Therefore, overexpression may be only at the injection site, without any circulating effect.
Using a highly sensitive PCR method to detect blood plasmid levels of HGF showed a slight increase in circulating levels at 1 day after the first injection and a similar increase after the second injection. However, this increase in plasmid levels rapidly disappeared, and there was no booster effect in plasmid DNA. Therefore, it is very safe. The level of circulating plasmid HGF was only 105 compared to the local level.
The ankle brachial index (ABI) was significantly increased from 0.47 to nearly 0.60 at 2 months and 0.67 at 6 months, and at 2 years it was 0.60 (p<0.05). Clinical symptoms gradually improved over the 2-year follow-up period. The mean resting pain was reduced from nearly 6 before gene therapy, to 3 at 2 months, about 1 at 6 months, and zero at 2 years (p<0.05). Similarly, ischemic ulcer was gradually healed, from a mean 3 cm before gene therapy to about a mean 0.6 cm at 24 months.
No difference in efficacy was seen between the ASO and TAO patients, with both having significant improvement in clinical symptoms and a function endpoint assessed by ABI.
Efficacy in the TREAT-HGF trial was not affected by the presence of various risk factors, including diabetes, hypertension, hyperlipidemia, smoking, and multiple complications. The injection site is the only parameter that might affect the outcome, and the site tended to correlate to improvement in ABI; injection below the knee seemed to be more effective than above the knee. However, because of the small number of patients, no firm conclusions can be made, but it does provide some indications of which patients can be treated with HGF.
Molecular mechanisms for HGF stimulation of angiogenesis
These investigators believe that HGF is a master gene to control angiogenesis through the essential transgene factor (Ets). HGF activates Ets, which drives multiple MMP, including MMP-1, -3, and -9). Interestingly, HGF activated endogenous HGF levels through its receptor c-met. Actually, an antisense ogliconucleotide against Ets results in complete inhibition of the over-induction of HGF levels induced by exogenous-activated HGF. Therefore, it may be possible that HGF acts as a positive feedback in the angiogenesis controls. In addition to the induction of HGF, it also induces another angiogenic growth factor, the vascular endothelial growth factor (VEGF) and its receptors. Again, the addition of antisense against Ets completely inhibited the upregulation of VEGF induced by HGF. Therefore, HGF works as an inducer of 2 important angiogenic factors, HGF and VEGF and their systems.
|
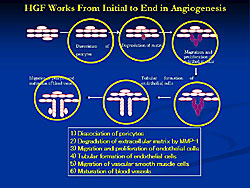
In addition to the characters of the master gene of HGF, they also identified how HGF works in angiogenesis (Figure 2). HGF worked from the dissociation of the surface and contributed to all pathways of this angiogenesis and ending in the maturation of the blood vessels. These unique characteristics might explain the potent angiogenic activity in our pilot studies.
Based on their pilot studies, they conducted 3 different trials to demonstrate HGF efficacy, including 2 double-blind, placebo-controlled trials. One was a Phase III double-blind, controlled study of 120 ASO patients conducted in Japan. Also, in Japan, they conducted open-label studies to treat TAO patients, including 15 inpatients. In the US, they conducted a Phase II double-blind, placebo-controlled studies to assess the safety and efficacy of HGF in peripheral ischemia.
HGF might be the first gene therapy drug to treat PAD patients in the world. However, there are limitations to therapeutic angiogenesis (Figure 3). The pharmacokinetics of the gene therapy drugs are the most important limitation.
HGF gene therapy to treat ischemic heart disease
In the porcine chronic infarction model as a preclinical study, using novel catheters, these investigators showed that the ischemic areas were significantly reduced in a dose-dependent manner at 1 month after intramyocardial injection of HGF. Similarly, improvement was seen in LVEF in a dose-dependent manner, assessed by both NOGA and echocardiography. These improvements in the myocardial ischemic areas were consistent with the increase in the myocardial blood flow, especially in the ischemic zones. Notably, a unique finding was that HGF inhibited fibrosis in the hamster and porcine models of myocardial ischemia compared to the placebo control animals. The frequency of arrhythmia was decreased after HGF therapy in animal models, in work done by Morishita and colleagues in collaboration with investigators from Ookayama University in Japan.
Therefore, HGF may be a very unique cardioprotective factor, with a potent ability to stimulate angiogenesis, anti-fibrotic effect, anti-arrhythmia effect, and an anti-apoptotic effect in cardiac myocytes, which was demonstrated by Matsuda and colleagues at Osaka University. Thus, HGF gene therapy may be a good tool to prevent myocardial infarction.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |