|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
|
|
Translational Research of Endothelin System: A Growing Body of Clinical Work Driven by High Quality Basic ScienceNoriaki EmotoKobe University Graduate School of Medicine
|
|
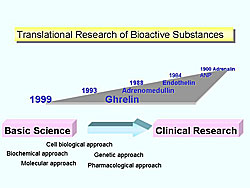
One of the first successful examples of translational research of bioactive substances is Dr. Jokichi Takamine, who more than 100 years ago isolated and crystallized adrenalin, the first pure hormone to be isolated from a natural source, which then was given a U.S. patent, Takamine was a biochemist and an industrial leader. Since then, Japanese researchers have contributed the findings of natriuretic peptide, endothelin, adrenomedullin, and ghrelin (Figure 1). Translational research connects basic science and clinical research.
The basic research findings with endothelin are now being applied in the clinical field, and thus provide a good example of translational research.
Review of basic science research
|
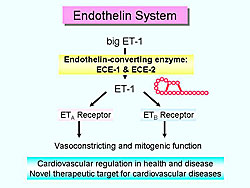
Endothelin-1 (ET-1) is a 21-amino acid peptide isolated from cultured porcine aortic endothelial cells and is a potent vasoconstrictor (Figure 2). ET-1 acts on two types of G-protein coupled endothelin receptors (ETA and ETB) and causes a variety of functions, including vasoconstriction and mitogenic. ET-1 is produced from the inactive precursor called big ET-1 by the action of ECE. To date, two endothelin-converting enzyme (ECE) family members have been identified: ECE-1 and ECE-2.
Endothelin has received considerable attention as a novel therapeutic target, especially among cardiovascular researchers, because of the potent vasoconstricting and mitogenic effects of the endothelin system.
|
Since the landmark discovery of ET was published in 1988, there have been more than 17,000 publications. Within the first year of its discovery, two structurally related peptides, ET-2 and ET-3, were isolated. In 1990, ETA and ETB receptors were isolated, leading to the identification of highly specific antagonists, BQ123 and bosentan, and the acceleration of related research. Notably, since the discovery of endothelin, the number of published papers in basic and clinical science has been similar, highlighting the benefits of effective collaboration between basic and clinical scientists.
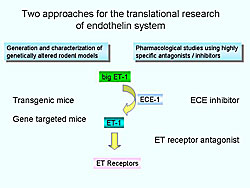
Two approaches for translational research of endothelin have been used (Figure 3). One is the generation and characterization of genetically-altered animals, and the other is pharmacological studies with highly-specific antagonists and inhibitors.
Translational studies with gene-knockout mice
|
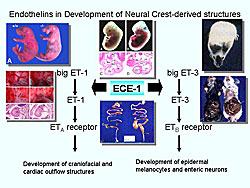
The importance of the endothelin system in development was dramatically defined through gene-knockout studies in mice (Figure 4). ETA- and ECE-1-knockout mice die at birth from mechanical obstruction of the airway due to severe malformation of craniofacial structures. These mice commonly display arterial defects and ventricular septum defects. ET-3- and ETB-knockout mice are viable at birth, but they suffer from aganglionic megacolon and subsequently die at 3-6 weeks of age.
Although novel insights were provided about fundamental embryology of neural crest development, the early deaths of the animals prevented physiological assessments in adult animals. Thus, several genetic tricks were used to avoid this problem.
Gene knockout study
One trick was to generate conditional knockout mice using cre-loxP recombinase system.
Using this method, Emoto and colleagues, in collaboration with Yanagisawa and colleagues characterized endothelial cell-specific ET-1 knockout mice. These mice develop normally, are healthy in adulthood, are fertile in both sexes, and live a normal life span. One finding in these mice was a 10 mmHg decrease in blood pressure, suggesting that ET-1 plays a distinct role in the physiological regulation of blood pressure.
Blood pressure elevation for 2 weeks is seen in mice treated with subcutaneous angiotensin II, the blood pressure elevated for 2 weeks. However, the blood pressure elevation in the second week is blunted in ET-1 deficient mice. Histological analysis demonstrated that vascular remodeling was attenuated, indicating that at least part of the effects by angiotensin II is induced through the endothelin system. Thus, endothelin blockade could be effective for this condition.
Pharmacological intervention
Endothelin antagonists are classified as ETA receptor or ETB receptor selective, or mixed antagonists. Many antagonists showed promising results in animal models of pulmonary hypertension, congestive heart failure, hypertension, and more recently several types of cancers. Some have progressed human trials.
Controlled clinical trials of endothelin receptor antagonists are being conducted in pulmonary hypertension, which is one of most promising target diseases.
One clinical trial with bosentan, an orally active, ETA-, ETB-mixed antagonist, showed that it significantly improves exercise capacity, symptoms and functional status in patients with pulmonary arterial hypertension. Based on the evidence from the clinical trials, bosentan received full marketing approval from the U.S. Food and Drug Administration for the treatment of patients with pulmonary hypertension. Bosentan is now recognized as a first-line therapy for patients with pulmonary arterial hypertension (PAH) in NYHA class III, rated with a Level of Evidence A.
Endothelin converting enzyme
To inhibit the action of ET-1, an ECE inhibitor could be an alternative approach. Emoto and colleagues studied the phenotype of gene-knockout mice. When exposed to hypoxic conditions, these mice develop pulmonary hypertension with right ventricular hypertrophy and pulmonary arterial remodeling. ECE-1 heterozygous knockout mice are completely resistant to this phenotype, suggesting that an ECE-1 inhibitor can be effective for PAH.
Then, they investigated the efficacy of an ECE-1 inhibitor to prevent hypertensive heart failure, and found the inhibitor had cardioprotective effects beyond blood pressure, similar to angiotensin-converting enzymes.
What’s next?
Despite the positive animal data, the efforts to translate therapeutic molecules to the clinical arena have been more problematic than anticipated. For the development of ET antagonists/inhibitors, several questions need to be answered. To inhibit the action of ET-1, ECE inhibitor could be an alternative approach. However, is an ETA selective or ETA- ETB-non-selective antagonist better? For an endothelin converting enzyme inhibitor, which is better—an ECE-1 selective or ECE-1/ACE dual inhibitor? What are the other optimal target diseases? The findings obtained from pharmacological studies and genetically-altered animal models will be applied clinical trials.
Clinical Trial of Human Gene Therapy Using Hepatocyte Growth Factor Gene TransferMotokuni AokiOsaka, University
|
|
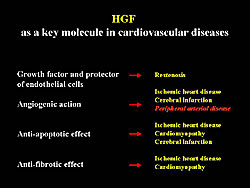
Therapeutic angiogenesis is expected to be a new treatment for patients with severe ischemic diseases. In the United States, VEGF and FGF may be used in gene therapy, and these have been suggested to have a beneficial effect. Work by Aoki and colleagues has focused on FGF, which they think can be a key molecule in cardiovascular diseases. It could be useful for the prevention of restenosis, because of HGF-induced re-endothelialization, and also be applied in ischemic diseases because of its powerful angiogenic action (Figure 1). Moreover, HGF has anti-apoptotic effects and anti-fibrotic effects on cardiomyocytes. Thus, cardiomyopthy could also be targeted with HGF.
TREAT-HGF Trial
Aoki and colleagues studied the use of intramuscular injection of naked plasmid DNA of HGF without a viral vector for the treatment of peripheral arterial disease (PAD). Despite the low transfection efficiency of naked plasmid DNA, they think that intramusclular gene transfer provided effective bioactivity because of the paracrine effects of the secreted gene product.
Men and women more than 40 years old with PAD and resting pain or ischemic ulcer (Fontaine IIb, III, IV), resting ABI < 0.6, and no response to conventional therapy were studied. Patients with a past history of cancer or proliferative diabetic retinopathy were excluded.
|
These Phase I and II non-randomized, open-label trials assessed the safety, efficacy, and dose response of HGF gene therapy. There were 2 stages in the trials, and each had a 4-week observation period, before gene injection to ensure that patient’s did not respond to conventional therapy.
In Stage 1, to test safety, in 6 patients a preliminary injection of 0.4 mg HGF plasmid was given, and then after checking for allergy, 2 mg injection was given. At 4 weeks, another injection of 2 mg plasmid HGF was given. In Stage 2, to evaluate dose response, a low and high dose (2 mg and 4 mg) was given at 4 and 8 weeks to 16 patients (8 in each dosing group).
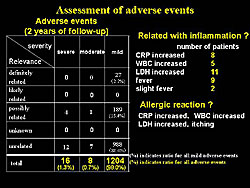
Assessment of adverse events at 2 years showed 1204 total adverse events, of which 189 (15.4%) were possibly related to gene therapy (Figure 2). Similar adverse events were suggested to be related to inflammation and 1 patient had what appeared to be an allergic reaction. However, there were no serious complications related to gene therapy. Thus, they concluded that the HGF plasmid was satisfactory.
Results at 2 months in TREAT-HGF
|
The safety of the transgene was also confirmed by the serum HGF level. An increase was not detected after intramuscular injection, and overexpression of HGF occurred only locally at the injection site. Thus, they believe that any influence on the remote organs or tissues can be excluded.
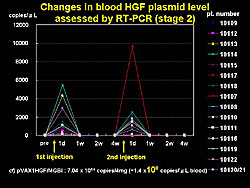
Another important safety parameter is leakage of plasmid into blood. A low level of plasmid was detected on the day after gene transfer, using RT-PCR, but it fell below the detectable level by 1 week in all cases (Figure 3).
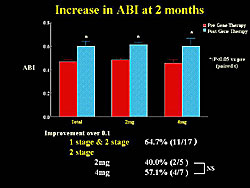
A significant increase in the ankle brachial index (ABI), a key parameter, was seen at 2 months after transfection. An improvement of >0.1 in the ABI was seen in 64.7% of patients (Figure 4).
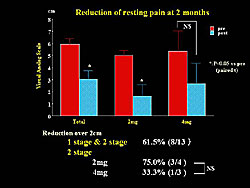
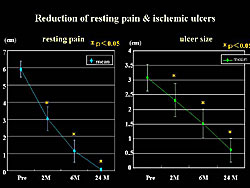
Reduction of resting pain, as assessed by the visual analog scale (VAS) was significantly reduced after 2 months of treatment. An improvement of > 2 cm was found in 61.% of patients (Figure 5).
|
|
Moreover, a significant reduction in ischemic ulcers was seen at 2 months after treatment, and 63.6% of patients had a reduction of 25% or more in the ischemic area.
|
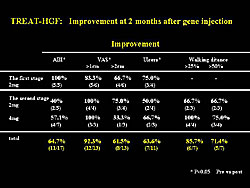
In summary, the main results of the TREAT-HGF trial showed that more than 65% had improved ABI, resting pain, or ischemic ulcers (Figure 6). In patients with claudication, a >50% improvement in walking distance was seen in 71.4% of patients. However, the lack of a control was a problem, and thus, a possible placebo effect may be present.
Regarding dose escalation, no significant difference was seen between the 2 doses of gene therapy at 2 months after transfection in this trial.
None of the background risk factors evaluated, including diabetes, hypertension, atherosclerosis, hyperlipidemia, smoking, and dialysis, affected the improvements seen with gene therapy. However, the injection site tended to correlate to an increase in ABI, with a greater improvement in ABI with below the knee injection compared to above the knee.
The 2-year results in TREAT-HGF
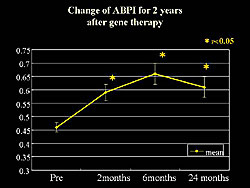
The significant increase in ABPI seen at 2 months was sustained
at 2 years after transfection (Figure
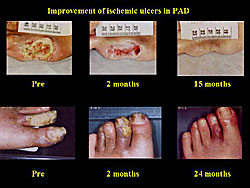
7). The improvement in ulcers was also seen at 2 years (Figure
8).
|
|
|
The reduction in resting pain and size of ischemic ulcers was also sustained at 2 years (Figure 9). The expression of the HGF gene is thought to disappear at 1 month, but these data suggest that the improvement was sustained for at least 2 years. The effect of gene therapy in the patients with claudication was sustained over 2 years. However, natural and historical improvement was often observed in patients with Fontaine Grade IIb, thus it is unclear whether this is surely the effect of the transgene.
Conclusions
No severe complication related to gene transfer occurred during the 2-year follow-up period in the TREAT-HGF trial. Gene therapy in this trial was well tolerated. Intramuscular gene transfer with HGF plasmid resulted in improvement of some parameters at 2 months after treatment. Moreover, this improvement was sustained over the 2-year follow-up.
The precise efficacy of gene transfer was not evaluated, because this was a Phase I/IIa trial. No evidence of gene expression was seen at the injection site. Therefore, they did not investigate the correlation between improvement of each parameter and local gene expression. Presently, there is no method for direct evaluation of microangiogenesis, not even by angiography. Nor is there a suitable parameter to demonstrate the effect of gene therapy. Improvement of clinical symptoms does not always correlate with objective examination findings, such as ABI, TPI, TCPO2, and laser Doppler.
Although there are some issues related to the evaluation, therapeutic angiogenesis seems to be highly practical. Presently, this is one of the most actively investigated fields worldwide. Some Phase III clinical studies are already underway. The clinical application of the HGF gene is now being investigated in a double-blind trial in 120 patients in 41 hospitals. If plasmid is shown to be effective in this Phase III trial, it will be possible to use genomic medicine in the near future, Aoki predicted.
Translational Research in Endothelial Progenitor Cell TransplantationTakayuki AsaharaTokai University School of Medicine
|
Asahara and colleagues investigated the CD34+ differentiation cascade
into endothelial cells in the peripheral blood, showing that endothelial
progenitor cells (EPCs) are derived from bone marrow, which can
recruit into the neovascularization site; the kinetics are regulated
by growth factors, cytokines, or hormones. These investigators and
others are working to isolate and deliver CD34+ cells into the neovascularization
site for therapeutic vasculogenesis.
This group showed that when total mononuclear cell transplantation is performed in acute myocardial infarction (AMI), hemorrhage occurs in the acute phase. However, no hemorrhage occurred with EPC transplantation of CD34+ cells. In nearly 10% of the cases, unexpected severe calcification occurred.
To move to the clinical setting, they studied the dose dependency (3 doses) of cell transplantation of human CD34+ cells (isolated from peripheral vascular patients) into the ischemic area of the nude rat MI model. Other than endothelial gene transcript, there was no myocardial or smooth muscle gene transcript in the initial population of CD34+ cells.
Findings from this work showed:
- Clear dose-dependent inhibition of LV remodeling after CD34+ cell transplantation (least amount of LV remodeling in the high-dose group)
- Clear dose-dependent recovery of LV function, using echocardiographic functional analysis
- Clear dose-dependent LV function, using a Millar micro-tip catheter for hemodynamic studies
- Dose-dependent human endothelial cell differentiation, by Unix staining
- Dose-dependent appearance of human smooth muscle cells
- A large amount of human-derived cardiomyocytes in the rat ischemic myocardium (unexpected)
- Dose-dependent rat myocardiogenesis
- Non-human BNP expressing myocardiocytes
- A large amount of dose-dependent human myocardiogenesis
- Dose-dependent rat angiogenesis by CD34+ cell transplantation
- Dose-dependent human-specific gene expression (myocardium, smooth muscle cells, endothelial cells)
In summary, CD34+ transplantation resulted in dose-dependent effects. No effect was seen with the low dose. The mid-dose was associated with vasculogenesis and myocardiogenesis, and these effects were even greater with the high dose.
Mechanisms
Transdifferentiation and cell fusion are two mechanisms being studied. Also, several findings indicate that there are some multipotent stem cells that can differentiate into the endothelial, smooth cell, or myocardial cells. There may be some multiple progenitor cells within this population.
Plasticity may differ, depending on the mechanism. For example, in the case of cell fusion, there is less plasticity, while there is more plasticity with the inclusion of the stem cells.
This group is working to define the transcription of each cell level, using single cell PCR. Although the results are very preliminary, they have identified some heterogenecity of CD34+ cells. This result indicates there is some vasculogenesis and myocardiogenesis at the mid-dose and high-dose. They defined that in vivo expansion methodology has an equivalent dose of a mid-dose. So, they moved to the clinical setting. They are using GCSF to mobilize and expand EPC. In that case, there is an enhanced number of EPC in the circulation, and it is possible to accumulate these cells by leukopheresis and EPC selection and transplant directly into the tissues.
Phase II clinical trial for PAD
This group conducted a clinical trial of the transplantation of autologous peripheral blood EPC (CD34+ cells) for vascular regeneration in patients with chronic critical limb ischemia. In this trial, GSCF administration, leukopheresis, and magnetic bead selection to isolate CD34+ cells was used, for direct transplantation into the leg of patients. For the dose evaluation study, the 3 doses are: low dose of 105 CD34+ cells/kg; mid-dose of 5x105 CD34+ cells/kg; high-dose of 106 CD34+ cells/kg.
Transplantation in 10 patients has been completed. In case 1, necrosis at the tip of one toe was improved at week 12. Pain scores were reduced in 4 cases by week 12, evaluated by the Wong Baker Faces pain resting scale. Absolute claudication distance and initial claudication distance on the treadmill was improved in the low and mid dose groups at week 4 and further improved at week 12. No serious adverse events have been reported in the initial 6 cases.
Asahara and colleagues are conducting a Phase II clinical trial of catheter-based autologous EPC transplantation for myocardial ischemia.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |