|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
PET Reporter Gene Imaging in the Myocardium: Monitoring of Gene Expression in Cardiac Angiogenic Gene TherapyMasayuki InubushiHamamatsu Medical Phototonics Foundation
|
Cardiac angiogenic gene therapy (AGT) has emerged as a novel treatment approach for patients with ischemic heart disease not amenable to conventional revascularization and medical therapy. AGT aims to facilitate neovascularization to augment blood flow in ischemic myocardium by introducing genes encoding for angiogenic factors, such as, VEGF and FGF.
|
Several clinical trials for cardiac AGT are underway, based on substantial encouraging preclinical results. However, few clinical trials have demonstrated constant therapeutic effects, due to technical inconsistency of gene delivery, individual variability in efficacy of gene expression, and, in some cases, confounding effects of concomitant revascularization therapy. Thus, the development of a noninvasive and quantitative method for evaluating gene transfer and expression is an important first step for AGT.
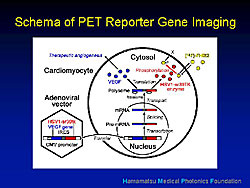
PET reporter gene imaging has been developed to monitor the expression of transgenes in vivo (Figure 1). The PET reporter can theoretically be linked with any therapeutic gene via the linker. Messenger RNA from the diffused gene can produce the same amount of the product from the two genes. If the FGF reporter probe is administered systemically via the vein, it can freely cross the cell membrane. But, in cells expressing viral kinase it is phosphorylated and unable to cross the cell membrane and is trapped intracellularly. Because the amount of F-HBG is proportional to the amount of viral kinase and accordingly the therapeutic product, the PET imaging with FHBG afforded thorough examination of location, magnitude, and duration of the expression of the therapeutic gene.
Proof of principle study
An adenovirus containing the promoter and the PET reporter gene (Ad-CMV-HSV-1-sr39tk), without any therapeutic gene, was injected directly at 2 or 3 sites of the anterolateral wall of the left ventricle during a thoracotomy.
|
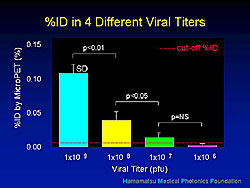
At day 3, imaging of myocardial perfusion with 13N-ammonia and of the gene expression with 18F-HBG was performed using microPET. Fusion imaging clearly showed the accumulation of F-HBG, in color, as well as the left and right ventricle. The accumulation of the F-HBG was consistent with the injected site. The kinases expressed in the area surrounding the needle track of the injection correlated well with the TK enzyme activity. Imaging sensitivity was assessed using different viral titers (Figure 2), and demonstrated the feasibility of the cellular imaging in the same subjects.
Application to monitoring gene therapy
Wu, Inubushi, and colleagues injected an adenovirus containing a VEGF isoform linked with the PET reporter gene via the second promoter (Ad-CMV-VEGF121-CMV-HSV1-sr39tk) at the peri-infarct region of rats that had undergone arterial ligation of the left anterior descending gene. MicroPET imaging was performed to assess the uptake of 18F-HBG by cells expressing the genes. Functional improvement was assessed from day 2 to week 10 using 13NH3 for myocardial perfusion and 18FDG PET for myocardial glucose metabolism, and ultrasound for myocardial contractility. Neovasculature was assessed by microscopic immunohistochemistry.
|
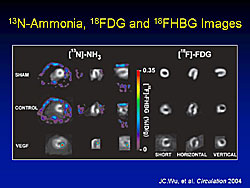
In the ammonia and FDG PET images, the MI in the anterolateral wall and the uptake defect are visible (Figure 3). In the study rats, the F-HBG accumulation is visible in the peri-infarct area, while there is no specific activity in the control rats.
Immunohistochemistry staining confirmed the reporter gene expression and the VEGF expression. In the same region, the capillaries stained with CD31 and small vessels with alpha-SMA stain were confirmed. Interestingly, the density of these microvessels was significantly higher than those in the control rats.
However, no significant improvement was seen in the functional assessments from day 2 to week 10 in the VEGF study rats or comparing the study and control rats, including the myocardial contractility by ultrasound, myocardial perfusion by ammonia, and metabolic glucose metabolism by 18FDG PET. It is likely that neovascularization was produced, but it did not translate into functional improvement.
Issues in AGT
A single therapeutic gene may not be sufficient to reproduce mature and functional microvessels. Physiological neovascularization is thought to be caused by the interaction of various angiogenic factors. Further understanding of the mechanism and the angiogenic cascade is required. Using multiple genes carried in one vector, a gene cocktail, may be useful for exerting synergic angiogenic effects. The use of master genes, such as hypoxia-induced growth factor (HIF-1alpa) may be better, whose expression activates and regulates various other genes in the angiogenic cascade and may lead to a broader and more physiologically balanced angiogenesis. Another possibility for the lack of functional improvement may be the relatively short period of gene expression by the adenovirus, which lasts about 2-4 weeks, likely due to host responses. So, less angiogenic vectors, including lentivirus, gutless adenovirus, and plasmid, may be better to prolong the duration of gene expression. Although they are poorer in transfection efficacy, compared with other viruses. PET reporter imaging will facilitate basic research to answer these questions.
Conclusions
- The principle of gene expression imaging using PET and reporter genes has been proved.
- PET reporter gene imaging can be used to monitor expression of therapeutic genes in-vivo in animals, and will facilitate designing strategies for AGT.
- If applied to humans, PET reporter gene imaging could aid in evaluating therapeutic effects of AGT.
- PET reporter gene imaging would play a critical part to identify the “missing link” in cardiac AGT as a powerful translational research tool.
Targeted Molecular Imaging and Therapy Using Ultrasound Contrast Agent MicrobubblesKatsuro TachibanaFukuoka University School of Medicine
|
The therapeutic use of ultrasound (US) was the focus of this lecture. Benefits of US, compared to other modalities, include: real-time imaging, relatively short and efficient imaging protocols, non-invasive with minima patient discomfort, and low operating costs. Recently, research with US has led to large expectations for using US for therapy, particularly drug delivery.
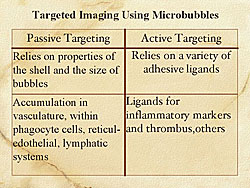
Passive targeting and active targeting of microbubbles are two possible approaches for targeted imaging. Passive targeting relies on the materials of the shell and the size of the microbubble (Figure 1). Active targeting relies on adhesive ligands, as markers of inflammation and thrombus, among other factors.
Microbubbles today have different characteristics that allow them to be used as targeting materials, including various sizes and the types of gases, and shell materials. Under development are microbubble agents, liposomal agents, and perflurocarbon emulsion nanoparticles. Disease and ligand targets are shown in Figure 2.
|
|
|
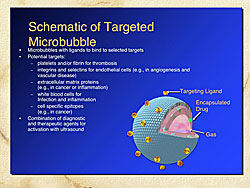
Figure 3 shows a schematic of a targeted microbubble. The microbubble is comprised of an outer shell, which can contain target ligands, and gases within the microbubble. Drugs can be encapsulated within the microbubbles, which can be targeted for drug release or enhancement of drug effects using US.
Hall and colleagues recently demonstrated nanoparticles that adhere to fibrin, and the nanoparticles can be visualized by US. Another example is a microbubble with GPIIb/IIIa receptor that adheres to thrombus, which has been demonstrated in an animal model. Lanza et al have shown that using nanoparticle accumulation and US imaging, it is possible to obtain enhanced imaging of balloon-injured arteries, whereas there was no enhancement in the control animals.
Tachibana and colleagues have been studying the therapeutic uses of US for nearly two decades. US alone enhances drug delivery and efficiency. Thrombolysis can be enhanced by the combination of US and thrombolytic agents. Tissue penetration can be enhanced by combining US and transdermal drug delivery. In 1995, microbubbles were first introduced, and this enhanced drug delivery into tissue. Gene therapy has been the main use for microbubbles in recent years.
In a 1989 study by Tachibana and colleagues, hairless mice exposed to US in Evan’s Blue stain had a significant increase of skin penetration of the stain, whereas there was little uptake in the control animals. The same mechanism can be used for thrombus. In another experiment, in 1992, using artificial clots they showed significant increases in fibrinolysis with combined urokinase and US irradiation compared to urokinase alone. The first specialized catheter for therapeutic use, which emits US energy simultaneously with urokinase and tPA, was approved by the United States Food and Drug Administration in 2004 for peripheral artery diseases.
|
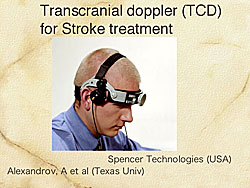
Alexandrov and colleagues have shown that transcranial Doppler (TCD) can expose US combined with urokinase through the skull in stroke patients, resulting in a 3-fold increase in the reocclusion rate in 126 patients in a randomized trial (Figure 4).
In the Netherlands, at Erasmus University, an ultra high-speed video imaging microscope has been used, which can obtain 25 million frames per second and observe 1 single microbubble. This videography shows that the microbubble repeatedly shrinks and enlarges. Also, the microbubbles can be collapsed with US. This method of preserving microbubbles has provided very detailed behavior of the microbubbles. Bursting of microbubbles in an asymmetric manner results in microjets that are high velocity but very small that can hit the cell from just one direction.
An experiment by Tachibana and colleagues in 1999, using scanning electron microscopy, showed that in a tumor cell treated by US, that a microbubble was thought to have burst near the tumor cells and thereby producing holes in the tumor cells. Any drug placed on the microjets that produce the holes will be carried into the cells. Tachibana and colleagues, in collaboration with Morishita et al, demonstrated this mechanism by using in vivo gene therapy experiments, using p53 plasmid non-viral vectors and US, and showed a decrease in restenosis. Also in collaboration with Morishita and colleagues, Tachibana’s group demonstrated enhanced angiogenesis with combined microbubbles and US.
A new approach being developed by ImaRx Therapeutics targets vascular blood clots by using microbubbles that adhere to thrombus, which are then exposed to US, thereby bursting the microbubble bursts and breaks up the clots.
|
In the future, it may be possible to inject into the lesion microbubbles or nanoparticles, perhaps using IVUS for diagnosis, and US for treatment to activate the targeted drugs. This may be an ideal method in cardiology.
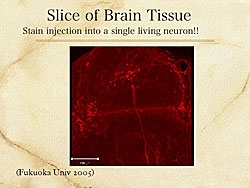
Ultra-selective-targeted drug delivery is a new concept being investigated by Tachibana and colleagues. Brain tissue from honey bees is exposed to microbubble and ultrasound stain injection into a single living neuron (Figure 5). This is only possible with microbubbles and US. Initially, this may be useful for research, but in time, echography targeted molecular contrast agents may be used for molecular diagnostics or molecular therapeutics.
Summary
Ultrasound is a promising modality for molecular imaging. Ultrasound contrast agent microbubbles can be used for therapeutic purposes, especially in thrombolysis and regenerative medicine. Future research will focus on the “fusion” of diagnostics and therapeutics.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |