|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Biocompatible and Absorbable Coating and StentsAlan C. YeungStanford University School of Medicine
|
Drug-eluting stents (DES) have been very effective. However, clinical concerns remain, despite the low thombosis rates of 1-2%. Residual thrombosis can lead to a large myocardial infarction or frequently death.
The thrombosis rates are quite similar with the Cypher, Taxus, and bare metal stents (BMS). However, the question is whether the thrombosis can be reduced close to zero, without taking antiplatelet drugs.
BMS are usually well covered by an intimal hyperplasia. But, with DES, because of the potency of the drug being eluted, sometimes struts are found that are thinly or barely covered by intimal hyperplasia. Hence, the concern is actually a “vulnerable” stent strut. The polymer around the metal of the strut is usually quite thin and usually next to the blood stream, providing the potential for some of the metal strut to be exposed to the blood stream.
The stent struts are comprised of the metal and the polymer, and over time the drug disappears (for example, with the Cypher stent) or some drug will remain (for example, with the Taxus stent). Thus, there is the potential for some metal, polymer, and drug to remain exposed to the blood stream. Using high-resolution imaging techniques, intimal hyperplasia is seen when looking at BMS in vivo. However, when looking at DES, it is possible to see, using an OCT with a resolution of 5-7 microns, that no material is covering a particular stent strut. Thus, there is the potential downstream for this to be a foreign body that potentially attaches thrombosis, in the absence of antiplatelet drugs. Further, there is the potential for incomplete apposition, either early, late, or at branches.
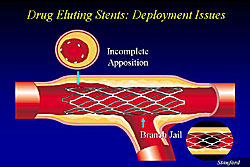
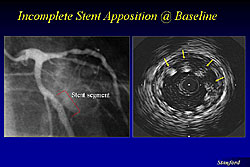
Figure 1 illustrates incomplete apposition of a stent, where the DES is placed near a side branch in the proximal area without focal dilatation and the side branch is being jailed. These are areas where the stent struts will potentially serve for a long time and are a nitus for thrombosis. Figure 2 illustrates incomplete apposition of the DES, from 11 o’clock to 3 o’clock.
|
|
The incidence of incomplete apposition increases, because of other possible issues, such as the lack of drug uptake by the blood vessel, and delayed endothelialization because the stent strut is not apposing the wall.
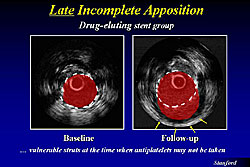
Late incomplete apposition also occurs, for example, because of positive remodeling that occurs from the high efficacy of the drug and thus pulls away from the stent strut. This provides the potential for areas of thrombosis, because the stent is now hanging in the lumen without any endothelial cells covering it (Figure 3).
Stent fracture that causes stress to the blood vessel can also be problematic (Figure 4). For example, an overlapping stent in the right coronary artery that was broken because of the pressure caused at end diastole. Thus, the struts of the stent can become a liability over time because of the potential issues of thrombosis, jailing side branches and breakage of the struts.
|
|
Solutions to decrease thrombosis
Phosphorylcholine (PC) coating is a polymer that mimics the human chemistry of the cell membrane surface. The PC polymer is biocompatible, because it has hydrophobic areas that stick to each other and to the metal, and also it is cross-linked for strength. Its high water affinity allows for water to be attracted to its surface. PC-coated devices have a permanent water layer on the surface, again serving as a potentially biocompatible surface.
|
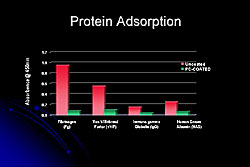
An uncoated device would have some thrombus and fibrin coating, but the PC-coated devices are clearly less attractive to blood cells and fibrin (Figure 5). These coatings do not seem to affect endothelialization, and within 5 days the device is covered with endothelial cells. Potentially, these types of coatings may enhance the safety of DES, because of the faster endothelialization and being more biocompatible.
In a baboon AV-fistula shunt model that tested PC-coated stents, platelet adhesion is much less with the PC-coated stent and no thrombus formation, while thrombus quickly formed on the uncoated stent. Biocompatible coatings are likely to be part of the future, in terms of trying to help the stents essentially heal by themselves without any antiplatelet therapy.
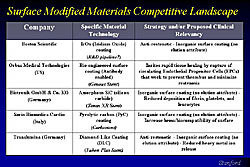
Bioabsorbable materials
Figures 6 and 7 list bioabsorbable materials being investigated. The Biosensors company is using polylactic acid as a bioabsorbable polymer and the drugs everolimus and biolimus. The safety of the materials have been shown in the FUTURE I FIH trial (everolimus) and the FUTURE II FIH trial (biolimus). Conor Medsystems is developing a unique design with wells drilled into it, and using polylactide/glycolide and polyanhydrides to absorb the drugs such as Paclitaxel and use it as a material to deliver the drug. This stent has been tested in Europe and India, and a trial will begin soon in the United States.
|
|
Other potential materials that may be more bioinert and biocompatible are also being studied. Boston Scientific is working on iridium oxide coating. for example. There are also other types of systems. For example, stents using a special-type of carbon coating, making the surface very inorganic. But, these may not have any elutive attributes.
To objective is to achieve a balance between using materials that can have drugs within them, elute the drugs, and have a surface material that can potentially coat the stent and make it more biocompatible.
Another approach is making the stent itself disappear (Figure 8). These might be made of a magnesium-based alloy that essentially disappears over about 6 months.
|
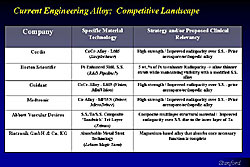
Programmable elution system (PES)
Another approach is not having any material on the surface. Stainless steel is very biocompatible in the sense that it has been used in many patients as a bare metal stent, with an extremely low thrombosis rate, and there is no need for increased use of antiplatelet drug.
|
In the PES, the eluting uses only metal (Figure 9). That is, the nanosurface has a certain amount of porosity that allows drug to be implanted into it and the metal itself serves as the eluting material. Thus, this is a stent made of stainless steel but has a nanotechnology surface on top, and the elusion characteristics can be changed to the mimic that of, for example, the Cypher stent. Further, there is no peeling of the surface and thus coming off the stent. Plus, there is very uniform drug elution, because of very even thickness at the acute angle.
Biodegradable, erodable stents
The holy grail is to develop stents that will disintegrate over 8-12 months. Such a stent would be present to add acute and subacute cross-sectional support that elutes a biological agent, and then disintegrates by 8-12 months later. And in the process of disappearing, it may elicit a biological response in the vessel wall, causing a plaque ceiling, with a thin layer of scar tissue or fibrosis that remains, preventing that part of the blood vessel to have further atherosclerosis, plaque buildup or plaque rupture. Thus, plaque stabilization may be a goal of this approach.
New Technologies in the Drug-Eluting Stent EraJiro AokiErasmus Medical Center
|
|
The use of drug-eluting stents (DES) is associated with markedly reduced restenosis in several randomized trials and registries, and they are now used in a majority of intracoronary stenting procedures. However, remaining issues include in-stent restenosis and late thrombosis after discontinuation of antiplatelet therapy has been reported.
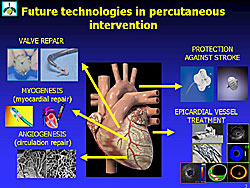
Other new approaches to treat coronary heart disease (CHD) include angiogenesis and myogenesis, valve repair and protection against stroke (Figure 1). Angiogenesis and myogenesis, the detection of vulnerable plaque, and imaging techniques were discussed in this lecture.
Angiogenesis and myogenesis
Angiogenesis. Therapeutic angiogenesis and vasculogenesis, which involves the administration of angiogenic growth factors, cytokines or stem cells to stimulate collateral formation and improve myocardial perfusion, is being tested as an alternative strategy for patients with medically intractable angina who are not candidates for mechanical revascularization therapies.
A variety of growth factors and chemokines convincingly increase the formation of small blood vessels in experimental models. Most clinical trials to date involve the transfer of vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) using several delivery strategies.
The efficacy of gene transfer approaches to therapeutic angiogenesis is now being tested in clinical trials. Controlled Phase II trials are providing positive but not definitive results. Gene therapy appears to be safe based on this data. Hard clinical endpoints, such as mortality, MI, and the need for revascularization are lacking, and also long-term follow-up.
Two large Phase III clinical trials (AGENT 3 and 4) evaluated the safety and efficacy of Ad5FGF4.26. These randomized, double-blind, placebo-controlled trials were terminated early in January 2004, because the interim data analysis of AGENT 3 indicated that the studies would not provide sufficient evidence of efficacy.
|
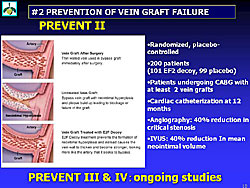
Vein graft failure. To prevent vein graft failure, a transcription factor decoy (very small pieces of DNA-like molecules) is being evaluated. The TF decoy turn off the function of target transcription factor. By blocking the E2F transcription factor, the E2F decoy treatment prevents the activation of the gene responsible for the proliferation of the smooth muscle cells that cause neointimal hyperplasia.Skeletal myoblasts. The E2f decoy prevents bypass vein graft failure. A 40% reduction in critical stenosis on QCA and 40% reduction in mean neointimal volume on IVUS were shown in the PREVENT II trial (Figure 2). Many other trials in this area are ongoing.
For the myogenesis of skeletal myoblasts, it was shown that these cells can survive in the myocardium when injected. One demonstration was in a patient who had died from cerebrovascular bleeding in whom skeletal myoblasts had been implanted 17.5 months before death, and another demonstration was in patients undergoing heart transplantation on average 122 days after the cell implantation. Although about 42 patients have been treated with skeletal myoblast therapy, improved symptom and LV function has been reported with this therapy.
Aoki and colleagues at the Thoraxcenter used pressure-volume (PV) loop to follow the 5 patients who have received skeletal myoblast therapy. The continuously monitored pressure-volume loops in steady state and during caval occlusion show dynamically the filling, contraction, ejection, and relaxation phase of the ventricle. A statistically significant improvement in the preload recruitable stroke work and the maximal rate of LV pressure increase (max dp/dt, mm HG) were found.
Bone marrow stem cell therapy. This is another approach being studied. Endothelial progenitor cells are known to transdifferentiate into myocytes in vitro. Willerson and colleagues showed in vivo that human peripheral CD34+ cells injected into immuno-depressed mice with subacute MI are incorporated into the vessel wall and myocardium.
Bone marrow-based cell therapy works in humans, as shown by data from 7 published studies. In the only randomized, controlled trial (BOOST), LV function was improved.
Resident myocardial stem cells. The myocardium has been shown to have its own stem cells. These stem cells are uncommitted. MEF2C is a transcription factor expressed early in the myocyte lineage, and C-KIT is an antigen of cell staminality (a growth factor called stem cell factor). The resident myocardial stem cells expressed C-KIT, proving the staminality potential, and expressed MEF2C, shows that some of the resident stem cells are still completely uncommitted, while a few are already committed towards myocyte lineage.
The multi-lineage capability of myocardial resident stems cells is the key finding so far, and will impact future research about what stem cells are the most appropriate for myocardial repair.
Imaging of vulnerable plaque
Sudden cardiac death remains a problem, occurring in one-half of all cardiac deaths; plaque rapture is the culprit in about 70% of these deaths. Novel invasive imaging techniques have been developed to better identify features of vulnerable plaque. It is hoped that better detection of high-risk plaques will help to prevent MI and sudden death.
Non-invasive diagnostic tools also can detect plaque vulnerability. For example, 64 MSCT coronary angiography, which can clearly detect the vessel lumen. MSCT has a 75% sensitivity and 73% specificity to detect plaque, compared to IVUS, in 5 mm subsegments.
|
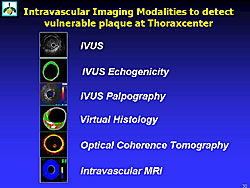
Figure 3 lists the intravascular imaging methods used by Aoki and colleagues to detect vulnerable plaque.
4D IVUS with ECG-gated acquisition detects the deformation of plaque during the cardiac cycle. Soft plaque easily changes the plaque shape. IVUS echogenicity is a computer-aided, gray-scale value analysis program to characterize plaque. Based on the mean gray level (brightness) of the adventitia, plaque are classified as more (hyperechogenic) or less bright (hypoechogenic) in relation to the adventitia.
The percentage of hyper- and hypoechogenicity can be detected. Hypoechogenicity indicates lipid-rich tissue and hyperechogenic tissue indicates fibrotic tissue.
IVUS palpography can detect the strain of the lesion. A high-strain lesion is soft, deformable, fragile, and breakable. It can also detect changes in vulnerability over the course of follow-up. Using IVUS palpography, Aoki and colleagues shows that in patients with ST-elevation MI, compared to stable patients, there are a large number of high-strain spots at baseline, but these are decreased at 6 months. They also showed a positive correlation between the incidence of high-strain spots and elevated CRP levels.
Virtual histology characterizes tissue based on the backscattering of radiofrequency signals, and can distinguish the lipid core, calcium, fibrofatty areas, fibrous tissue, and the media.
Optical coherence tomography (OCT) is a new class of ultra-high resolution imaging technology that uses high-bandwidth light sources, fiber optics and advanced signal processing. OCT has 10x better resolution than IVUS. Aoki and colleagues are evaluating the use of OCT elastography to image the cap and to assess the mechanical properties of the vulnerable plaque. For example, it is possible to easily distinguish soft and stiff tissue using an OCT image of Cryogel Phantom at alternating pressures.
Intravscular MRI can be used to index arterial wall lipid infiltration. Water diffusion is decreased within the atherosclerotic (lipid) plaque compared with the fibrous cap and medial smooth muscle layer. Within fibrous material water diffusion is almost unrestricted. In lipid material with restricted diffusion of the water molecules, an MR signal will decay quickly, but in fibrous material it will decay slowly. The extent and location of increased vascular lipid infiltration can be used to determine the presence of a thin fibrous cap with increased luminal lipid deposition overlying a lipid-rich necrotic core, creating a uniquely detailed anatomic and histologic diagnosis of the presence of a thin-cap fibroatheroma.
|
|
| Copyright © 2005 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |