|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
State of the Art: Novel Nanotechnology to Evaluate Gene Expression in Living CellsGang Bao, PhDDepartment of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, Georgia, USADr. Gang Bao, Georgia Institute of Technology and Emory University, provided an overview of nanotechnology and its potential uses in medicine. Nanotechnology is the development of engineered nano-scale (1-100 nm) structures and devices for improved diagnostics and highly specific medical interventions. The three major areas under development are: engineering nanocarriers and nanodevices for targeted therapy; studying molecular nanomachines and protein complexes in living cells; and developing probes for molecular imaging. Dr. Bao is lead investigator of a study using nanotechnology for detection and molecular analysis of plaque formation. Technologies under development in this study include: molecular beacon probes for genome targeting; quantum dots for studying protein-protein interactions inside living cells and detecting plaque in small animals; magnetic nanoparticles for plaque detection in vivo; polymer-based nanoparticles for targeting therapy; and quantum dot probes or molecular beacons for targeting reactive oxygen species within cells.
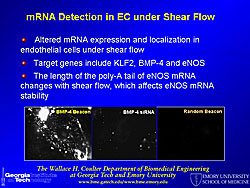
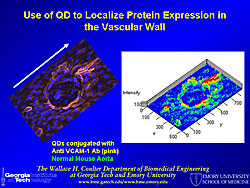
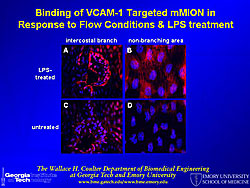
A molecular beacon probe targets a specific mRNA sequence inside cells, using fluorescence for localization. Different types of mRNA have different appearances and are localized in different parts of the cell. A molecular beacon probe was used to study shear stress-induced changes in KLF2 mRNA in endothelial cells (Figure 1). Photos show the signal for BMP-4 localized by the beacon, BMP-4 knocked down by siRNA, and a random beacon control. Quantum dots (QD) for fluorescence imaging have the following properties: 20 times brighter, 1,000 times more stable, and 3 times narrower spectral linewidth than organic dyes; size and material tunable emission; simultaneous excitation; and narrow symmetric emission. QD tagged to VCAM-1 antibody were used to localize and show the distribution of VCAM-1 in the mouse vascular wall (Figure 2). Multiple epitopes can be targeted at the same time, using a different color for each epitope. QDs were also used to quantify the expression level of VCAM-1 in aortic endothelium, using LPS to increase and an inhibitor to decrease VCAM-1 expression. A multifunctional micelle coated magnetic iron oxide nanoparticle (mMION) probe was developed from an iron oxide core coated with phospholipid. Different targeted ligands, radiolabeled ligands, and/or fluorescent dyes can be added to the coating for use with MRI, PET, or fluorescence imaging. Use of a VCAM-1 targeted mMION shows response to flow conditions and LPS treatment in the intercostal branch and a non-branching area (Figure 3). According to Dr. Bao this technology has great potential for human use because iron oxide is non-toxic. Dr. Bao concluded that quantum dots, magnetic nanoparticles, and molecular beacons can be used for sensitive detection and molecular analysis of plaque formation. |
Real-Time Two-Photon Imaging of Ischemia/Reperfused HeartsMasaharu AkaoDepartment of Cardiovascular Medicine, Kyoto University Graduate School of Medicine, Kyoto, JapanMitochondria play a central role in cell death during myocardial ischemia/reperfusion. The collapse of mitochondrial membrane potential (delta-psi) is the earliest event committing the cell to death. However, visualizing delta-psi in the heart has been difficult because the heart is thick and composed of highly light-scattering tissue. Additionally, mitochondria are susceptible to photodamage. Dr. Masaharu Akao, Kyoto University Graduate School of Medicine, described how these obstacles were overcome by use of two-photon laser scanning technology (TPLSM). TPLSM uses a much longer wavelength (700-800 nm) than conventional one-photon excitation, allowing deeper tissue penetration and less photodamage.
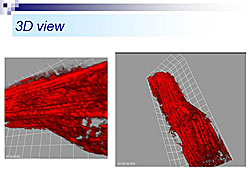
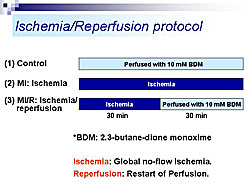
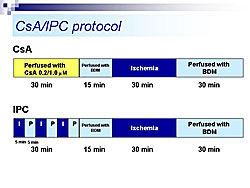
Rat heart was perfused using the Langendorff method, in the presence of tetramethylrhodamine ethyl-ester (TMRE), a fluorescent indicator of delta-psi. Using TPLSM, TMRE fluorescence in cardiac cells was visualized up to about 60 µm from the epicardial surface. For the first time, mitochondria stained by TMRE were visualized. At high power, single mitochondria (2-3 µm) could be observed. Three-dimensional images of a single cardiac myocyte show many mitochondria along the myofilaments (Figure 1). Using this system, delta-psi changes were monitored in hearts subjected to perfusion (control), ischemia (MI), or ischemia followed by reperfusion (MI/R) (Figure 2). Time lapse images taken every five minutes showed that control hearts remained stable. In the MI group, no-flow ischemia caused progressive delta-psi loss in the cardiomyocytes. The delta-psi level remained constant for up to 20 minutes but suddenly collapsed within five minutes. In the MI/R group, the cardiomyocytes had progressive delta-psi loss during ischemia, followed by a rapid, complete, and irreversible collapse, but this process did not affect neighboring cells. At the end of reperfusion there was a remarkable cellular heterogeneity in delta-psi level, which could cause arrhythmias after ischemia and reperfusion. The time course of delta-psi loss was almost the same for ischemia and reperfusion, suggesting a common underlying mechanism. Images taken every 10 seconds showed that delta-psi loss was initiated in a particular area of mitochondria and rapidly propagated along the longitudinal axis within the cell. Hearts subjected to ischemia and reperfusion were treated with either cyclosporin A (CsA), a mitochondrial PT pore blocker or ischemic preconditioning (IPC), an endogenous cardioprotective mechanism (Figure 3). Time-lapse images showed that both CsA and IPC had protective effects, but the kinetics of delta-psi loss were different suggesting different mechanisms of action. According to Dr. Akao, this real-time TPLSM imaging system for monitoring mitochondrial function could be valuable for developing a more integrated understanding of ischemia/reperfusion injury and deeper insights into anti-ischemia/reperfusion therapy targeting mitochondria. |
Nanotechnology for Cardiovascular Diagnosis and TherapySamuel A. WicklineCenter for Translational Research in Advanced Imaging and Nanomedicine, Washington University, St. Louis, Missouri, USADr. Samuel A. Wickline, Director of the Washington University Center for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN), discussed C-TRAIN’s experience developing nanoparticles for molecular imaging of atherosclerosis and delivering drugs to the vascular wall.
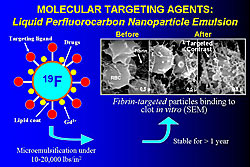
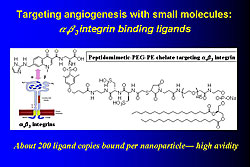
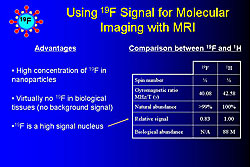
In the mid 1990s, C-TRAIN invented liquid perfluorocarbon nanoparticle emulsions to which a variety of targeting ligands, drugs, and other substances such as gadolinium (Gd), fluorescent molecules, and radionuclides can be attached. For example, these nanoparticles were used to target fibrin particles in vitro (Figure 1). Adding Gd to a nanoparticle allows imaging with MRI. From 50,000-100,000 Gd molecules can be added to each paramagnetic nanoemulsion particle, producing a very bright image with a relaxivity (r1) of >106 compared to 4, which is used in conventional imaging. These constructs can be used to visualize thrombi with MRI in living animals using a fibrin targeted molecule. Angiogenesis, which plays a key role in early atherosclerotic lesions, can be targeted with small molecules such as αvß3integrin binding ligands (Figure 2). For example, angiogenic capillaries have been clearly imaged in this way in the aorta of cholesterol-fed rabbits and in very small rabbit tumors. MRI imaging can also be done with ultrasmall iron oxide particles (~18 nm), which are taken up by aortic macrophages and can be targeted to molecules such as VCAM. Another method is use of fluorine (19F), a high signal nucleus that can be used in high concentrations in nanoparticles and does not produce a background signal (Figure 3). Fluorine imaging of unstable plaque can be used to produce a quantitative mapping of fibrin binding sites. Nanoparticles can deliver large payloads of drugs that suppress angiogenesis, reduce restenosis, or inhibit inflammatory processes in the vascular wall. Drug is delivered by a unique mechanism of interaction between nanoparticles and cell membranes involving vesicle-cell fusion followed by lipid mixing and drug delivery directly to the cell cytoplasm without the need for endocytosis. In one study, αvß3targeted nanoparticles loaded with the antiangiogenic drug fumagillan were used to treat rabbit atherosclerosis. One week after treatment with a single dose there was almost no αvß3 left compared to no change in control animals. The same technique was used to treat tumors in rabbits. Two weeks after treatment the tumors had shrunk by 58% and the area of angiogenesis had decreased by 82%. Nanoparticle technology is a new method of targeted drug delivery that has the potential to both reduce plaque burden and interrupt processes that lead to plaque instability, rupture, and infarction. |
|
|
|
Copyright © 2007 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |