|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Establishment of Pentraxin 3 (PTX3) ELISA System for the evaluation of Acute Coronary SyndromeKenji InoueDepartment of Cardiology, Juntendo Nerima Hospital, Tokyo, JapanPentraxin 3 (PTX3) is a potential biomarker for identifying patients with acute coronary syndrome (ACS). PTX3 belongs to the same family as C-reactive protein (CRP) and is primarily expressed in endothelial cells, macrophages, and dendritic cells. Dr. Kenji Inoue, Juntendo Nerima Hospital, Tokyo, found that PTX3 is suppressed by pitavastatin in endothelial cells. He subsequently investigated PTX3 as a marker for cardiovascular disease (CVD) using a highly sensitive ELISA system developed in his laboratory. Using this ELISA in 73 volunteers without a history of CVD, the average PTX3 was 1.92 ± 0.95 ng/mL, with a range from about 1.50 ng/mL to 2.75 ng/mL. PTX3 measurements were normal in 423 cardiovascular patients stratified by CVD risk factors, indicating that PTX3 is not associated with CVD risk factors.
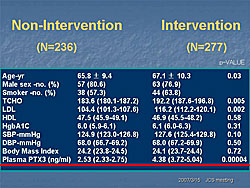
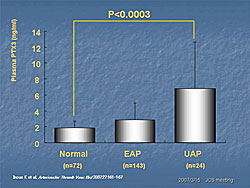
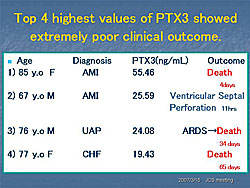
PTX3 levels were analyzed in 630 patients with CVD seen at Juntendo Hospital for coronary artery evaluation. The mean PTX3 was 3.38 ng/mL (95% CI 3.06-3.70). PTX3 levels were significantly higher in the patients who had interventions (N=277, 4.38 ng/mL) than in those who did not have an intervention (N=236, 2.53 ng/mL) (P=0.00004) (Figure 1). PTX3 was higher in patients with effort angina pectoris (EAP) and significantly higher in patients with unstable angina pectoris (UAP) than in subjects without angina (P<0.0003) (Figure 2). According to Dr. Inoue, PTX3 meets the criteria for a CVD risk predictor established by the 2002 CDC/AHA Workshop in Inflammatory Markers and CVD. These studies show that PTX3 is an ideal marker for unstable angina. The standardized ELISA system allows controlled measurement and the established normal values guide interpretation of results. PTX3 is an independent predictor associated with CVD clinical endpoints in observational and clinical studies. The prognostic value of PTX3 is being assessed in patients admitted to the emergency department for chest pain. So far, 67 patients have been evaluated. Of these, the four patients with the highest PTX3 values (19.43–55.46 ng/mL) have had very poor clinical outcomes (Figure 3). Because of the small number of patients, it is difficult to draw conclusions from these preliminary data. Dr. Inoue concluded that the PTX3 assay system developed at his hospital is highly specific. The plasma PTX3 level is increased in patients with acute atherosclerosis but not in those with chronic, or stable, atherosclerosis. Thus, the PTX3 level may reflect the degree of stability of a patient’s atherosclerotic plaque. |
Sex Differences in Long-Term Prognosis of Japanese Patients with Acute Myocardial InfarctionHiroshi OgawaDepartment of Cardiology, Tokyo Women’s Medical University, Tokyo, JapanNumerous observational studies have reported higher total mortality from acute myocardial infarction (AMI) in women than in men. In a recent report, age-adjusted hospital mortality was higher for women and associated with a lower rate of percutaneous coronary intervention (PCI) (Circulation 2007;115:833). Another study found that in patients undergoing primary stenting for AMI, women had higher hospital mortality rates than men (Circ J 2006;70:217). However, after adjusting for baseline differences female sex was not independently associated with increased mortality. The objective of this study, presented by Dr. Hiroshi Ogawa, Tokyo Women’s Medical University, was to compare the differences in long-term prognosis in men and women after AMI.
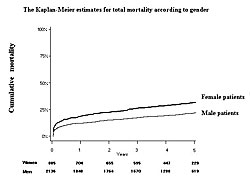
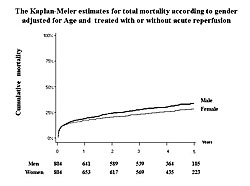
A total of 3,021 consecutive patients (885 women; 2,136 men) admitted to hospitals with AMI between January 1999 and June 2001 were followed for a mean of 40 months. Of these patients, 1,451 (48%) received primary PCI. During the follow-up period, the cumulative mortality rate was higher in women than in men (Figure 1). When adjusted for age, there were no significant differences in mortality rates between men and women who were ≥80 years old, 70-79 years old, or under 70 years old. Analysis of baseline characteristics showed that the mean age of the women was 74±10 compared with 65±12 for the men (P<0.01). Women also had significantly higher rates of hypertension (P<0.001) and heart failure (P<0.001). Only 54% of women underwent acute reperfusion compared with 70% of men (P<0.001). Significantly fewer women than men were prescribed concomitant medications upon discharge (P<0.001 for ACE-I or ARBs, beta blockers, Ca-channel blockers, nitrates, and aspirin). After adjusting for age and acute reperfusion, men had a higher risk of death over the entire follow-up period (HR 1.21, P=0.031) (Slide 9). The total long-term mortality rate also was higher in men after adjusting for age and acute reperfusion (Figure 2). These results show that advanced age and underutilization of proven beneficial therapies correlate with poorer outcomes in women with AMI. However, after adjusting for age and whether patients were treated or not treated with reperfusion, the long-term outcome is significantly better in women than in men. Dr. Ogawa concluded that the onset of AMI is delayed in women by about 10 years compared to men. Thus, further improvements in therapy for AMI in elderly patients are essential. |
Important Role of Placental Growth Factor on the Healing Process and Survival after Acute Myocardial InfarctionShiro UemuraFirst Department of Internal Medicine, Nara Medical University, Kashihara, Nara, JapanBone marrow derived progenitor cells have an important role in the healing process after acute myocardial infarction (AMI) by enhancing angiogenesis and tissue reconstruction. Dr. Shiro Uemura, Nara Medical University, investigated the role of placental growth factor (PLGF) as a mediator between the injured myocardium and bone marrow cells. PLGF is a vascular endothelial growth factor (VEGF) that stimulates angiogenesis and activates monocytes through selective binding of VEGF receptor-1 (Flt-1) expressed on blood cells. A clinical study found that PLGF levels are significantly elevated in peripheral blood on the third hospital day after AMI compared to controls (P<0.0001). Peripheral blood levels rapidly increase with reperfusion and then gradually decline to baseline within seven days. Before reperfusion, PLGF levels are the same in the coronary artery and coronary sinus, but after reperfusion levels are significantly higher in the coronary sinus. Increased peripheral PLGF levels positively correlated with increased numbers of circulating CD34+ progenitor cells (P<0.05). Additionally, elevated PLGF was the strongest independent predictor for restoration of left ventricular ejection fraction (LVEF) in the chronic phase (P<0.05). Based on the results of the clinical study, Dr. Uemura’s group investigated the cardiac PLGF mRNA increases in mouse models of AMI. PLGF gene transcription was rapidly and significantly increased within 24 hours after AMI (P<0.01) and returned to normal in seven days. PLGF protein was overexpressed primarily in coronary artery endothelial cells in the infarct region. Next the investigators studied the effects of recombinant PLGF (rPLGF) on survival and healing after AMI in mice. Treatment with rPLGF resulted in a 52% improvement in survival 28 days after coronary ligation compared with control mice treated with peripheral blood stem cells (PBS) . Areas of myocardial infarction were reduced in the acute phase and there was less scar formation in the chronic phase of MI in the rPLGF-treated mice. Treatment with rPLGF also stimulated CD31+ vascular endothelial cells and α-smooth muscle actin (αSMA) positive cells, indicating both angiogenesis and arteriogenesis in the infarcted areas. Further analysis showed that rPLGF stimulates mobilization and homing of bone marrow derived cells in mice with AMI. Dr. Uemura concluded that PLGF is an important factor involved in the healing process after AMI. Recombinant PLGF treatment preserves left ventricular function and survival rates in mouse models of AMI. Therefore, rPLGF has the potential to become a novel treatment for AMI. |
|
|
|
Copyright © 2007 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |