|
|
||||||
|
|||||||
|
|||||||
|
|||
|
|||
Plaque Stabilization by Angiotensin II Signal Blockade that Inhibits Differentiation and Proliferation of Macrophage ProgenitorsHiroyuki YamadaDepartment of Cardiovascular Medicine, Kyoto Prefectural University School of Medicine, Kyoto, JapanAngiotensin II promotes the formation of atherosclerotic plaque through its actions on endothelial cells, smooth muscle cells, monocytes/macrophages, lymphocytes, and platelets. The mechanism by which angiotensin II acts on macrophage progenitors in the bone marrow (BM) is unclear. Dr. Hiroyuki Yamada, Kyoto Prefectural University School of Medicine, reported results of experiments on the effects of angiotensin II signal blockade on macrophage progenitor proliferation and plaque stabilization. In the first experiment, BM cells from angiotensin 1a deficient (AT1a-KO) or wild type (WT) mice were transplanted into BM-ablated apoE-KO mice, producing apoE-KO/BM-Agtr1+/+ mice and apoE-KO/BM-Agtr1-/- mice. The atherosclerotic lesion area was reduced by 60% in the apoE-KO/BM-Agtr1-/- mice compared with the apoE-KO/BM-Agtr1+/+ mice (P<0.05) (Slide 4). Accumulation of MOMA-2+ monocytes/macrophages was also reduced in the apoE-KO/BM-Agtr1-/- mice (P<0.05). The numbers of monocytes in the BM and the circulation were significantly reduced in the apoE-KO/BM-Agtr1-/- mice compared with the apoE-KO/BM-Agtr1+/+ mice (P<0.05). The number of circulating monocytes expressing CCR2 and exhibiting migratory activity was significantly reduced in apoE-KO/BM-Agtr1-/- mice compared with apoE-KO/BM-Agtr1+/+ mice (P<0.05). The apoE-KO/BM-Agtr1-/- mice also had significantly reduced hematopoietic stem cell (HSC) transition to monocyte/macrophage progenitors.
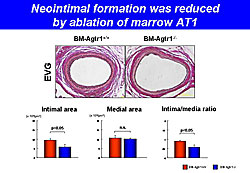
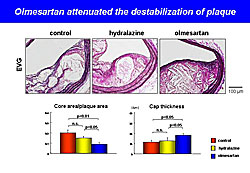
In the second experiment, BM cells from AT1a-KO or WT mice were transplanted into ablated WT mice, producing BM-Agtr1+/+ and BM-Agtr1-/- mice. Blood vessel examination after arterial injury showed that neointimal formation was significantly reduced in the BM-Agtr1-/- mice compared with the BM-Agtr1+/+ mice (P<0.05) (Figure 1). Accumulation of intimal MOMA-2+ cells also was significantly reduced in the BM-Agtr1-/- mice (P<0.05). BM-Agtr1-/- mice with arterial injury had significantly fewer circulating monocytes than BM-Agtr1+/+ mice (P<0.05). After arterial injury, the BM-Agtr1-/- mice had fewer circulating c-Kit-Sca-1+lin- cells (P<0.05) and intimal Sca-1+ cells than BM-Agtr1+/+ mice (P<0.05). In the third experiment, apoE-KO mice were fed a Western diet for 19 weeks and received olmesartan, hydralazine, or no treatment from week 15 through 19. After 19 weeks, the olmesartan-treated mice had a significantly smaller plaque area than hydralazine-treated mice (P<0.01) and untreated mice (P<0.01). Olmesartan significantly increased plaque stabilization compared with hydralazine and control (Figure 2). The numbers of circulating monocytes, macrophage progenitors, and circulating inflammatory monocytes were significantly reduced in the olmesartan group compared to the other groups. BM-AT1 is strongly associated with the development of HSCs into macrophage progenitors and with monocyte/macrophage behavior in atherosclerotic lesions. These results suggest that BM-AT1 is a potential therapeutic target for the prevention of cardiovascular events. |
Essential Role of c-Jun N-Terminal Kinase in Destructive Vascular RemodelingHiroki AokiDepartment of Molecular Cardiovascular Biology, Yamaguchi University School of Medicine, Ube, JapanUnstable atheromatous plaque is characterized by infiltration of macrophages that secrete proinflammatory mediators. Activation of matrix metalloproteinases (MMPs) leads to collagen and elastin degradation and destabilization of the fibrous cap, resulting in degradation of the extracellular matrix (ECM). Unstable plaque and abdominal aortic aneurysm (AAA) share many pathological features, including inflammatory signaling and ECM degradation. Dr. Hiroki Aoki, Yamaguchi University School of Medicine, presented results of studies on the role of c-Jun N-terminal kinase (JNK) in destructive vascular remodeling. Currently, no drug therapies are available to correct AAA. Possible drug targets in AAA include the growth factors, inflammatory mediators, and stress signals that play a role in the chronic inflammatory process. Among possible drug targets for AAA is the stress signal factor, JNK. When activated, JNK-dependent genes in vascular smooth muscle cells promote ECM destruction through upregulation of MMP-9 and inhibit ECM repair through suppression of biosynthetic enzymes, including prolyl 4-hydroxylase (P4H), lysyl hydroxylase, and lysyl oxidase (Lox) in vascular smooth muscle cells. The investigators used periaortic application of CaCl2 to create a mouse model of AAA. MMP-9 levels increased and Lox levels decreased early after CaCl2 administration. Next, CaCl2 was applied to wild type (WT), JNK1-knockout (KO), and JNK2-KO mice. AAA developed in the WT and JNK1-KO mice, but not in JNK2-KO mice. The effect of JNK inhibition was tested in mice treated with CaCl2 for 6 weeks, after which the mice were treated with either vehicle or JNK inhibitor from week 6 to week 12. The aortic diameter increased after CaCl2 administration, then decreased substantially in mice treated with JNK inhibitor but only slightly in mice that received vehicle. Histological examination at 12 weeks showed that tissue repair was enhanced and the inflammatory response suppressed in the JNK-treated mice compared with the vehicle-treated mice. AAA was induced in ApoE-/- mice by angiotensin II infusion for 4 weeks. Either JNK inhibitor or vehicle was administered from week 4 to week 12. Aortic diameter decreased substantially in the mice administered JNK inhibitor and increased slightly in mice administered vehicle. These results show that selective inhibition of JNK prevents macrophage infiltration, ECM degradation, and AAA development in mouse models. JNK inhibition after establishment of AAA enhanced tissue repair, normalized ECM architecture, and caused regression of aortic aneurysm. These results may also be relevant to stabilization of atheromatous plaque, which shares many molecular features with AAA. |
Chondromodulin-I Maintains Normal Cardiac Valvular Function by Preventing AngiogenesisKeiichi FukudaRegenerative Medicine and Advanced Cardiac Therapeutics, Keio University School of Medicine, Tokyo, JapanNeovascularization and expression of angiogenic growth factors and their receptors have been observed in cardiac valvular disease, suggesting that the normal cardiac valve avascularity was disrupted. The purpose of this study, presented by Keiichi Fukuda, Keio University School of Medicine, was to clarify the role of the angiogenesis inhibitor, chondromodulin-I (Chm-1) in maintaining valvular function. Chm-1 is expressed in avascular tissues. Analysis of rat and human tissues found that Chm-1 is expressed in the cartilage, eyes, and cardiac valves. Immunostaining of adult mouse hearts found that Chm-1 is specifically expressed in the cardiac valves. The investigators analyzed the cardiac valves of Chm-1-/- mice to determine if the valves maintain their vascularity and if they exhibit valvular disease. They found that vascular endothelial growth factor (VEGF) was expressed in the cardiac valves of Chm-1-/- mice but not in the valves of Chm-1+/+ mice. Further, valvular thickening, ectopic calcification, and lipid deposition were observed in the Chm-1-/- mouse valves but were absent in the Chm-1+/+ valves. The Chm-1-/- mice also had bulky aortic valves and turbulent blood flow in the aortic valve area by Doppler echocardiography, while the Chm-1+/+ mice did not. Rat valvular interstitial cells (VICs) were cultured and observed to express Chm-1 in the culture medium. When added to a culture of human coronary artery endothelial cells (HCAEC), the rat VIC-conditioned culture medium significantly inhibited tube formation in the HCAEC compared to controls (P<0.001). HCAEC migration was also inhibited when the HCAECs were co-cultured with rat VIC medium. Expression of Chm-1 and VEGF were analyzed in the cardiac valves of humans with rheumatic heart disease, infective endocarditis, atherosclerotic valvular disease, and without valvular heart disease (VHD). Histologic analysis showed that Chm-1 was expressed in the normal cardiac valves but VEGF was not. In contrast, Chm-1 expression was markedly decreased in the patients with infective endocarditis, rheumatic heart disease, and atherosclerotic valvular disease. VEGF was strongly expressed and neovascularization was observed in the diseased valves. In summary, these studies show that Chm-1 is expressed in normal mouse, rat, and human cardiac valves. Cardiac valves in Chm-1-/- mice exhibit neovascularization and early valvular heart disease. Chm-1 secreted from vascular interstitial cells inhibits tube formation and migration in human coronary endothelial cells. Chm-1 was downregulated and VEGF and neovascularization were observed in patients with valvular heart disease. Dr. Fukuda concluded that Chm-1 maintains normal cardiac valvular function by preventing angiogenesis. |
|
|
|
Copyright © 2007 Japanese Circulation Society All Rights Reserved. webmaster@j-circ.or.jp |