 |
|
|
 |
IS058
Diabetes, PAI-1, and Atherogenesis |
|
David J. Schneider, M.D.
Cardiovascular Division
University of Vermont
Burlington, VT, USA |
|
|
|
 |
|
|
 |
|
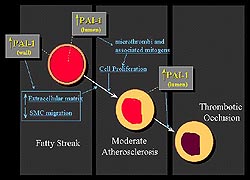 |
Figure 1.
Schematic of the relation between PAI-1 and atherogenesis.
In the vessel lumen, PAI-1is critical in the thrombolytic
response to vascular injury. In the vessel wall, PAI-1
appears to be critical in atherogenesis by impairing
cell-surface proteolysis, mediated primarily by urokinase
type plasminogen activator (u-PA). (Schneider 2000)
Click to
enlarge |
|
The relation between PAI-1 and atherogenesis
can be conceived in terms of two compartments, the lumen
and the vessel wall (Fig. 1). In the vessel lumen, PAI-1,
a primary physiologic inhibitor of plasminogen activators,
both tissue and urokinase type, is critical in the thrombolytic
response to vascular injury. Increased PAI-1 diminishes
the fibrinolytic response and consequently a larger thrombus
burden and a greater risk of thrombotic occlusion is expected.
Further, persistence of thrombi can accelerate the process
of atherosclerosis by exposing the vessel wall to clot-associated
mitogens.
In the vessel wall, PAI-1 appears to
be critical in atherogenesis by impairing cell-surface
proteolysis, mediated primarily by urokinase type plasminogen
activator (u-PA). Impaired proteolytic activity can potentiate
accumulation of extracellular matrix and, importantly,
may limit migration of smooth muscle cells (SMC). Paradoxically,
this decrease in SMC migration may actually potentiate
the genesis of vulnerable plaques. Limiting SMC migration
into the neointima and the cap overlying plaques may lead
to the formation of more thin, acellular fibrous caps
as opposed to more cellular caps. Acellular caps are more
prone to rupture.
|
PAGE
TOP
Increased
plasma levels of PAI-1 |
|
| |
PAI-1 is increased by 3- to 4-fold
in blood from patients with type 2 diabetes and
obese subjects with insulin resistance, as shown
by studies in Schneider's laboratory. The increased
concentration of PAI-1 was associated with increased
functional activity of PAI-1. This increased expression
of PAI-1 is associated with a diminished fibrinolytic
response. Patients were exposed to a physiological
stress (venous occlusion) and the change in tPA
activity was measured to determine fibrinolytic
response. A decreased fibrinolytic response was
found in the obese (1.8 IU/ml) and diabetic (1.5
IU/ml; p<0.05 vs lean) subjects compared to the
lean subjects (2.7 IU/ml).
|
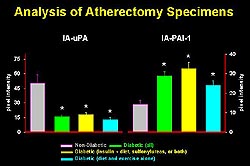 |
Figure
2. Atherectomy specimens from diabetic and non-diabetic
subjects showed increased immunoreactivity of
PAI-1 in samples from diabetics, regardless of
the type of diabetic treatment. This increase
in PAI-1 was associated with a decrease in uPA.(Circulation
1998;97:2213-2221.)
Click
to enlarge
|
|
Analysis of atherectomy specimens
from diabetic and non-diabetic subjects showed increased
immunoreactivity of PAI-1 in samples from diabetics,
regardless of the type of diabetic treatment (Fig.
2). This increase in PAI-1 was associated with a
decrease in uPA. These results in association with
their observations made in PAI-1 deficient mice
led them to suggest that the overexpression of PAI-1
combined with the decreased fibrinolytic activity
in the vessel wall may potentiate the genesis of
unstable plaques.
|
|
PAGE
TOP
PAI-1, lipids
and insulin |
|
The infusion of glucose and triglycerides
into healthy subjects increased concentrations of
insulin and PAI-1 in blood. By contrast, the infusion
of insulin and glucose with the use of euglycemic
clamp techniques led to a decrease in the concentration
of free fatty acid, while the concentration of triglycerides
remained low throughout the infusion. As expected,
insulin levels rose and glucose was maintained normally
but the concentration of PAI-1 was similar to that
seen in subjects in whom normal saline was infused.
These experiments strongly support that an interaction
between lipids, particularly triglycerides and free
fatty acids, glucose, and insulin modulates expression
of PAI-1 in diabetic subjects.
In vitro studies with a human hepatoma
cell line (HepG2 cells) support this observation.
In the HepG2 cells, exposure to insulin alone and
free fatty acids alone led to a modest (2-fold)
increase in PAI-1. Combining insulin and free fatty
acids resulted in a synergistic increase in the
expression of PAI-1. Exposure to insulin and free
fatty acid concentrations similar to those seen
in diabetic subjects led to a 3- to 4-fold increase
in the accumulation of PAI-1 in conditioned media
|
|
PAGE
TOP
Studies of
molecular mechanisms |
|
| |
The molecular mechanisms by which
insulin alters expression of PAI-1 was characterized
in HepG2 cells. Nuclear run-on assays demonstrated
that insulin does not increase the rate of transcription
of PAI-1. The degradation of PAI-1 mRNA was determined
after transcription was inhibited with actinomycin
D. Insulin prolongs of the half-life of PAI-1 mRNA.
Thus, insulin-mediated increased expression of PAI-1
in HepG2 cells is post-transcriptional.
|
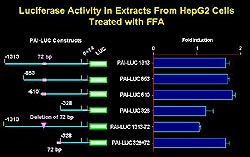 |
Figure
3. Free fatty acids increased the luciferase activity
nearly 2-fold when HepG2 cells were transfected
with the chimeric gene construct with approximately
1300 base pairs of the 5'-flanking DNA inserted
upstream to a luciferase reporter. The fatty acid
responsive element is located upstream of -610.
Deletion of a 72-base pair segment led to abolition
of the response to free fatty acids, and when
this 72-base pair segment was placed upstream
of a minimal promoter the response to free fatty
acids returned. (Schneider 2000)
Click
to enlarge |
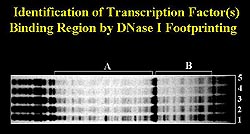 |
Figure
4. Dnase 1 footprinting identified a specific
sequence that is repeated four times in the 72-base
pair segment that contains a fatty acid responsive
region. (Schneider 2000)
Click
to enlarge |
|
Free fatty acid studies
A chimeric gene construct was created
in which approximately 1300 base pairs of the 5'-flanking
DNA were inserted upstream to a luciferase reporter.
Free fatty acids increased the luciferase activity
nearly 2-fold when HepG2 cells were transfected
with the construct (Fig. 3). A promoter-deletion
analysis showed that the fatty acid responsive element
was located upstream of -610.
Deletion of a 72-base pair segment
from the full-length construct of the 5'-flanking
PAI-1 DNA led to abolition of the response to free
fatty acids. Further, when this 72-base pair segment
was placed upstream of a minimal promoter the response
to free fatty acids returned. Thus, the 72-base
pair segment contains a fatty acid responsive region.
Gel shift mobility assays and Dnase
1 footprinting identified a specific sequence that
is repeated four times in the 72-base pair segment
(Fig. 4). Substantial structural homology between
the repeated sequence and an SP-1 binding site exists.
However, super shift assays with an anti-SP-1 antibody
did not demonstrate a 'supershift'. Thus, further
studies are underway to identify the SP-1 like transcription
factor that mediates the effect of PAI-1.
|
|
PAGE
TOP
|
Diabetes is clearly associated with
an increased concentration of PAI-1 in the blood
and in the arterial wall. Increased PAI-1 may potentiate
atherosclerosis by promoting thrombosis (limiting
fibrinolysis) and by impairing vessel wall proteolytic
capacity that alters the composition of the plaque.
Potential mechanisms responsible for increased expression
of PAI-1 in diabetic subjects include hyperinsulinemia,
hyperlipidemia, and hyperglycemia. Insulin-mediated
effects in HepG2 cells are secondary to stabilization
of PAI-1 mRNA. Free fatty acids increase transcription
of PAI-1 mRNA. The combination is synergistic.
|
|
PAGE
TOP
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2000
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|
|