Nerbonne reviewed work in her laboratory aimed at understanding
the molecular basis of the functional diversity of the
voltage-gated potassium (K+) channels that
underlie action potential repolarization in the mammalian
myocardium. In addition, she discussed the electrical
remodeling that occurs in the heart when the functional
expression of repolarizing voltage-gated K+
channels is altered.
|
PAGE
TOP
Multiple
classes of voltage-gated K+ channel currents |
|
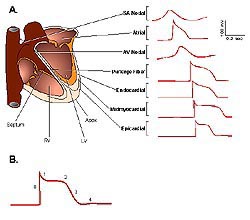 |
Figure
1. Action potential waveforms and propagation
in the human heart. (A) Schematic of action potentials,
recorded in different regions of the human heart,
are displaced in time to reflect the temporal
sequence of propagation. (B) Schematic of a ventricular
action potential labelled as follows: (0) depolarization;
(1) early (fast) repolarization; (2) plateau phase;
(3) late (slow) phase of repolarization; and,
(4) after hyperpolarization/return to the resting
membrane potential. (SA, sino-atrial; AV-atrio-ventricular;
RV, right ventricle; LV, left ventricle) (Journal
of Physiology 2000;525(2):285-298).
Click
to enlarge |
|
Multiple voltage-gated K + currents
have been identified in the mammalian myocardium ( Table 1).
These currents are differentially expressed and contribute
to the marked variations in the waveforms of action
potentials in different regions of the heart (Figure
1). Two broad classes of voltage-gated K +
channel currents contributing to the repolarization
phase of the action potential are:
 |
Transient outward currents,
Ito (Table 1) |
|
Importantly, the properties of the various currents
(Ito,f, Ito,s, IKr,
IKs and IKur, etc.; see Table 1)
in different cardiac cell types and in different
species are remarkably similar (Table 1),
suggesting that the molecular correlates of these
currents - which have been distinguished electrophysiologically
and pharmacologically - in different cell types
are also the same. However, this remains to be proven.
|
| |
|
|
PAGE
TOP
Identifying
potential molecular correlates |
|
There is considerable interest in
exploring the molecular mechanisms that regulate
the functional expression of voltage-gated K+
channels in the myocardium under physiological and
pathophysiological conditions. Pivotal to this effort
has been recent studies aimed at identifying the
molecular correlates of the different types of voltage-gated
K+ channels in cardiac cells.
The first voltage-gated K+
channel pore-forming (a) subunit was cloned
from the Shaker locus in Drosophila.
The Shaker protein has six transmembrane domains,
a highly charged S4 region that underlies the voltage-dependent
gating properties of the channel, and a region between
the fifth and sixth transmembrane domains that underlies
the K+ selected pore. A number of C and
N terminal splice variants of Shaker have
been identified that give rise to K+
currents with distinct properties. Three additional,
homologous genes, Shal, Shab and Shaw,
which also encode voltage-gated (Kv) a subunits
were subsequently cloned from Drosophila.
Heterologous expression of these subunits also gives
rise to voltage-gated K+ currents, albeit
with distinct time- and voltage-dependent properties.
|
|
PAGE
TOP
|
Molecular cloning techniques have
been used to identify a number of mammalian homologues
of Shaker, Shal, Shab and Shaw and importantly, in mammals, there are many members
of each subfamily. Kva subunits of the Shaker subfamily are referred to as Kv1, and the genes
are distinguished as Kv1.1, Kv1.2, and so forth
(Table
2). The Shab subfamily is called Kv2,
the Shaw subfamily Kv3, and the Shal
subfamily Kv4 (Table
2). Functional voltage-gated K+ channels
comprise four subunits and, in heterologous expression
systems, members of the same subfamily can combine
to form K+ channels with time and voltage-dependent
properties different from the homomeric channels
formed by the individual subunits alone. The role
of heteromultimeric Kva subunit assembly in
the generation of functional voltage-gated cardiac
K+ channels, however, is unclear.
Additional, homologous subfamilies
of Kva subunits,
called ERG and KvLQT, have also been identified
in the myocardium (Table
2). Importantly, ERG1 is the locus of mutations
in long QT syndrome type 2 and mutations in KvLQT1
underlie long QT syndrome type 1. MiRP, and others
are cytosolic, such as ß, KChIP and KChAP,
proteins.
|
|
PAGE
TOP
Experiments
to identify subunits underlying channels |
|
Molecular genetics is powerful tool for defining
the roles of the various Kva
subunits in the generation of functional voltage-gated
K+ channels, as is evident in the case
of ERG1, which underlies cardiac IKr,
and KvLQT1, which underlies cardiac IKs.
In the case of the other cardiac K+ currents,
however, alternative experimental strategies are
necessary to define these relationships. Work in
Nerbonne's laboratory, for example, has focussed
on exploiting in vivo approaches in mice,
using transgenic and targeted deletion strategies,
to identify the molecular correlates of the transient
outward K+ currents, Ito,f and
Ito,s.
|
|
PAGE
TOP
Transient
outward currents |
|
| |
Comparison of the properties of heterologously
expressed Kva subunits
and cardiac transient outward currents and analysis
of the expression levels of the various Kva
subunits in the myocardium, led to the hypotheses
that KV4 a
subunits underlie Ito,f and that Kv1.4
underlies Ito,s. These hypotheses were
tested in vivo using transgenic and targeted
deletion strategies.
Kv4 alpha subunits underlie Ito,f
To test directly the hypothesis that
Kv4a subunits underlie
Ito,f, Nerbonne and colleagues generated
transgenic mice expressing a mutant Kv4.2 (Kv4.2W362F)
subunit that functions as a dominant negative. In
contrast to wild type Kv4.2 or Kv4.3, heterologous
expression of Kv4.2W362F alone does not reveal voltage-gated
K+ currents. When Kv4.2W362F is coexpressed
with Kv4.2 or Kv4.3, however, the (wild type) Kv4.2-
or Kv4.3-induced currents are eliminated or markedly
reduced. Therefore, the mutant subunit functions as
a dominant negative. Critically, this association
was demonstrated to be subfamily specific.
The Kv4.2W362F construct was placed
behind the a myosin heavy chain promoter to direct
cardiac specific expression of the transgene, and
several lines of transgenic mice were developed. Electrophysiological
experiments revealed that Ito,f is eliminated
in ventricular and atrial myocytes isolated from the
Kv4.2W362F-expressing transgenics, demonstrating directly
that members of the Kv4 subfamily underlie Ito,f
in both (mouse) atria and ventricles. Marked
action potential prolongation occurs in Kv4.2W362F-expressing
cells, and surface ECG recordings revealed QT prolongation
in these animals.
|
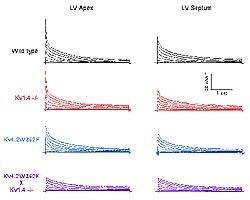 |
Figure
2. Molecular genetic dissection of the transient
outward K+ currents, Ito,f
and Ito,s in mouse ventricular myocytes.
Representative outward K+ current waveforms
recorded from adult C57BL6 mouse left ventricular
(LV) apex and septum cells in response to 4.5
sec depolarizing voltage steps to -20 mV to +50
mV from a holding potential of -70 mV. Records
from wild type, Kv4.2W362F-expressing, Kv1.4-/-
and Kv4.2W362F x Kv1.4 -/- LV cells are illustrated.
(Nerbonne 2000)
Click
to enlarge |
|
Electrical remodeling
In contrast to the dramatic effect
on Ito,f, the densities and the properties
of the other prominent voltage-gated outward K+
currents, IK,slow and Iss,
in mouse ventricular cells are unaffected by Kv4.2W362F
expression (Figure 2). Detailed analysis of the
currents, however, suggested the presence of an
additional, "novel" current component with decay
kinetics slower than Ito,f and faster
than IK,slow in wild-type cells. Although
this slow transient current could reflect effects
of the mutant Kv4.2W362F on the properties of the
wild type (Ito,f) channels, this seems
unlikely given that no effects on kinetics are seen
when the mutant subunit and wild-type Kv4 a
subunits are co-expressed in heterologous systems.
Rather, it appeared that a slow transient outward
current was upregulated in Kv4.2W362F-expressing
ventricular cells. Biochemical experiments revealed
that Kv1.4 protein expression is increased in the
ventricles of the Kv4.2W362F-expressing animals,
whereas Kv1.2 and Kv2.1 protein expression levels
in these animals are not significantly different
from those determined in wild type animals. These
results suggested a role for Kv1.4 in the generation
of the slow transient K+ current in Kv4.2W362F-expressing
cells.
Kv1.4 underlies Ito,s
Subsequent experiments revealed marked
heterogeneity in the waveforms of the voltage-gated
outward K+ currents in different regions
of the mouse left ventricle (LV). In cells from
the LV apex, for example, Ito,f is prominent
and IK,slow and Iss are also
expressed (Figure 2). The waveforms of the currents
in cells isolated from the LV septum, however, are
distinct (Figure 2). In most (~ 75 %) of the (septum)
cells, IKslow, Iss and Ito,f
are expressed, although the density of Ito,f
in these (LV septum cells) is significantly lower
than in apex cells. In addition, in the septum cells
lacking Ito,f, a slow transient current,
referred to as Ito,s, was identified.
Subsequent analyses revealed that Ito,s
is expressed in all septum cells, i.e., in septum
cells with Ito,f and in septum cells
lacking Ito,f. Importantly, Ito,s
is not detected in wild type apex cells. In
addition, the properties of Ito,s in
wild type septum cells are indistinguishable from
the slow transient current described above that
was identified in Kv4.2W362F-expresing transgenics
cells. Interestingly, further experiments revealed
that this current is only upregulated in Kv4.2W362F-expressing
apex cells; Ito,s density is not increased
in septum cells isolated from the Kv4.2W362F-expressing
animals.
To determine the role of Kv1.4 in
the generation of Ito,s, electrophysiological
experiments were completed on ventricular myocytes
isolated from animals with a targeted deletion in
Kv1.4 (Kv1.4-/- animals). These experiments revealed
that Ito,s is undetectable in (all) septum
cells from the Kv1.4-/- mice (Figure 2). The properties
and densities of the other currents, Iss,
IK,slow and Ito,f in septum
(and apex) cells, however, were unaffected by the
loss of Kv1.4 (Ito,s). In addition and
in contrast to the Kv4.2W362F-expressing transgenics,
no electrical remodeling was seen in the Kv1.4-/-
septum (or apex) cells.
To test the hypothesis that Kv1.4
upregulation underlies the appearance of slow transient
current in Kv4.2W362F-expressing apex cells, they
expressed Kv4.2W362F in the Kv1.4-/- background.
Voltage-clamp recording from ventricular myocytes
isolated from these animals revealed that Ito,f
and Ito,s are eliminated (Figure
2). The waveforms of the currents in LV septum and
apex cells from the crossed (Kv4.2W362F x Kv1.4-/-)
animals are remarkably different from those recorded
from wild-type LV cells (Figure 2). Interestingly,
the currents in LV apex and septum cells from the
crossed animals look remarkably similar when Ito,f
and Ito,s are both eliminated (Figure
2), suggesting that the ventricles will have become
remarkably homogeneous in terms of repolarization.
In addition, analyses of the waveforms of the outward
currents in the cells from the crossed animals revealed
only the presence of IK,slow and Iss.
Thus, in contrast to the Kv4.2W362F-expressing transgenics
in which electrical remodeling is seen in (LV apex)
cells when Ito,f is eliminated, there
is no evidence of remodeling when Ito,f
and Ito,s are both eliminated (Figure
2). Marked action potential prolongation, however,
is seen in the cells isolated from the crossed animals
and, in some cases, early after depolarizations were
also noted.
|
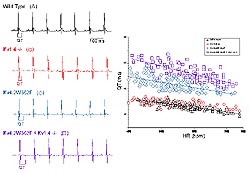 |
Figure
3: QT prolongation in mice lacking Ito,f,Ito,sor
both Ito,f and Ito,s.Left
panel: Telemetric ECG recording were obtained
from conscious adult C57BL6 mice with the genotypes
indicated. Right panel: Variations in QT intervals
with heart rate in wild type, Kv4.2W362F-expressing,
Kv1.4-/- and Kv4.2W362F x Kv1.4 -/- LV cells are
illustrated. (Nerbonne 2000)
Click
to enlarge |
|
Functional consequences of elimination
of Ito,f and Ito,s
To examine the consequences of manipulating
the expression of functional voltage-gated K+
channels in vivo, Nerbonne and colleagues
obtained telemetric electrocardiographic (ECG) recordings
from the Kv4.2W362F-expressing, the Kv1.4-/-, and
the crossed mice, and compared these with ECG recordings
from wild type animals (Figure 3). Comparison of QT
intervals in the various lines of mice revealed that
QT intervals vary with heart rate in all of the animals
and that, in the Kv1.4-/- animals, QT intervals are
not significantly different from those in wild type
(Figure 3), indicating very little effect of elimination
of Ito,s. In the animals expressing the
mutant Kv4.2 transgene with Ito,f eliminated,
there is marked QT prolongation (Figure 3). In addition,
QT intervals are further prolonged in the crossed
animals, which lack both Ito,f and Ito,s
(Figure 3). These results suggest that the upregulation
of Ito,s in apex cells plays a role in
the Kv4.2W362F-expressing animals to limit the impact
of the elimination of Ito,f and that, when
Ito,s cannot increase (in the Kv1.4-/-
background, the more dramatic functional consequences
of the loss of Ito,f are revealed (Figure
3).
|
|
PAGE
TOP
Closing Remarks/Future
Directions |
|
A number of the other subunits have
been shown by Nerbonne and other investigators to
contribute to other types of voltage-gated channels.
The functional roles of nearly all of the Kv a
subunits that encode the various types of cardiac
voltage-gated K + channels have been identified
( Table
2). Notable exceptions are Kv1.7 and Kv 2.2, whose
roles are presently unknown. A role for the Kv3 subfamily
has recently been suggested in the generation of the
ultrarapid component of outward rectification, I Kur,
in the canine myocardium, whereas Nerbonne's lab and
the laboratory of Stanley Nattel in Montreal have
shown that in both humans and rats, Kv1.5 underlies
I Kur. This is clearly an exception to the
general rule that the various currents in different
cell types and species are so similar it can be assumed
the same subunits underlie them. In contrast to the Kva
subunits, less is known about the functioning of voltage-gated
K+ channel accessory subunits. For example,
although a number of cytosolic Kvß subunits
have been identified in heart and shown to affect
the expression and the properties of Kva
subunit encoded channels in heterologous expression
systems, the role of these (Kvß) subunits in
the myocardium is not known. Similarly, the accessory
proteins, KChAPs and KChIPs, have been shown to increase
the functional expression of cell surface voltage-gated
K+ channels in heterologous expression
systems. The roles of these proteins in the normal
physiology or in the pathophysiology of the myocardium,
however, are unknown. In the nervous system, considerable
evidence has accumulated demonstrating that ion channels
and neurotransmitter receptors are anchored in the
membrane through interactions with the cytoskeleton.
It has recently been suggested that voltage-gated
K+ channels in the myocardium interact
with similar anchoring proteins and/or cytoskeletal
elements and that these interactions also play roles
in regulating functional K+ channel expression
and/or properties in cardiac cells. Defining the roles
of Kv accessory proteins and interactions with the
cytoskeleton will likely be active and important areas
of future research.
|
|
PAGE
TOP
Report
Index | Previous Report
| Next Report
Scientific
Sessions | Activities
| Publications
Index
Copyright © 2000
Japanese Circulation Society
All Rights Reserved.
webmaster@j-circ.or.jp
|